Abstract
Much of the forest remaining in South East Asia has been selectively logged. The processes promoting species coexistence may be the key to the recovery and maintenance of diversity in these forests. One such process is the Janzen–Connell mechanism, where specialized natural enemies such as seed predators maintain diversity by inhibiting regeneration near conspecifics. In Neotropical forests, anthropogenic disturbance can disrupt the Janzen–Connell mechanism, but similar data are unavailable for South East Asia. We investigated the effects of conspecific density (two spatial scales) and distance from fruiting trees on seed and seedling survival of the canopy tree Parashorea malaanonan in unlogged and logged forests in Sabah, Malaysia. The production of mature seeds was higher in unlogged forest, perhaps because high adult densities facilitate pollination or satiate pre-dispersal predators. In both forest types, post-dispersal survival was reduced by small-scale (1 m2) conspecific density, but not by proximity to the nearest fruiting tree. Large-scale conspecific density (seeds per fruiting tree) reduced predation, probably by satiating predators. Higher seed production in unlogged forest, in combination with slightly higher survival, meant that recruitment was almost entirely limited to unlogged forest. Thus, while logging might not affect the Janzen–Connell mechanism at this site, it may influence the recruitment of particular species.
Keywords: Janzen–Connell hypothesis, logging, plant diversity, anthropogenic disturbance, predator satiation, secondary forest
1. Introduction
Tropical forests are being lost and degraded at an alarming rate [1,2]. Forest loss has been particularly rapid in South East Asia, where less than half of the original forest cover remains [3]. While unexploited forest is now rare in the region, large areas of secondary forest remain [4,5] and their importance for conservation is increasingly recognized [6–8]. Commercial timber extraction has inevitable, dramatic impacts on the structure, diversity and community composition of these secondary forests [9,10], but they are still important reservoirs for biodiversity and provide crucial ecosystem services including provision of raw materials, soil protection, and sequestrating and storing carbon [8,9,11,12].
Despite the importance of secondary forests for species conservation, the consequences of human disturbance for the maintenance of diversity remain poorly understood [13,14]. While there is an extensive literature on the effects of human disturbance on species richness and diversity (see recent reviews in [7,8]), the effects on the structure and organization of ecological communities and associated ecological functions and processes have received less attention [13,14]. In particular, for forest biodiversity to recover and persist following disturbances such as logging, it is important that the processes responsible for maintaining diversity remain intact [14]. The mechanisms maintaining tree diversity have been extensively examined in the literature [15], and these mechanisms could be potentially disrupted by disturbance [14]. Reductions in tree diversity could have implications for diversity in other taxonomic groups because plant diversity, to some extent, sets the template for diversity at higher trophic levels [16,17]. A particular concern is that, even when diversity is not severely reduced by exploitation itself, it might decline over time if these processes have been undermined.
Much research has tried to understand the processes involved in plant species coexistence, particularly in tropical forests [15]. Among the most likely candidates is the Janzen–Connell mechanism [18,19], where seeds and seedlings near conspecific adults or in areas of high conspecific density suffer high mortality through the activity of specialized natural enemies. Consequently, locally rare species have an advantage, promoting species coexistence.
Support for the Janzen–Connell mechanism mainly comes from the Neotropics, and studies from Asia and Africa are rare [20]. Of the small number of studies conducted in Asia, several support the Janzen–Connell mechanism [21–23] but others do not and sometimes show patterns that run counter to its predictions [24,25]. The ecology of South East Asian forests differs in several important regards from other tropical regions, and so we need to be cautious when generalizing from results obtained elsewhere. In particular, reproduction is typically highly episodic in these forests, occurring in supra-annual, community-wide mast fruiting events [26,27]. Up to 88 per cent of canopy species can fruit at the same time and the most plausible explanation for this phenomenon is that it evolved to satiate seed predators [27,28]. Predator satiation decreases mortality at the highest densities, directly opposing the Janzen–Connell mechanism. Indeed, Janzen [18] suggested that mast fruiting species were unlikely to be affected by the mechanism. However, while much of the literature on fruiting in South East Asian forests has concentrated on mast events, there is some evidence of successful recruitment outside mast events [25,29]. In these partial fruiting episodes, the chance of observing density dependence may be greater because fruiting trees are relatively isolated. Seed predators may be attracted to these trees, decreasing the survival of seeds close to their parent trees relative to those dispersed further away.
Deforestation and hunting may reduce seed predator populations, which may then be unable to constrain the recruitment of common species. Conversely, restricting predators to small forest fragments could prevent seeds of rare species from escaping in space, removing the rare species advantage. Both scenarios would weaken the Janzen–Connell mechanism. Studies in the Neotropics have suggested that human disturbance disrupts the Janzen–Connell mechanism. Hunting and forest fragmentation have both been linked to reduced density dependence and consequently plant diversity [30–32]. However, similar data are lacking for South East Asian forests, although forest fragmentation and logging can reduce dipterocarp recruitment because low densities of dipterocarp trees are unable to produce enough seed to satiate seed predators (which are localized in remaining forest fragments) [26,33].
In this paper, we assess the distance from the nearest fruiting conspecific tree (which, in most cases will be the parent) and conspecific density effects on the survival of Parashorea malaanonan Merr (Dipterocarpaceae) seeds and germinating seedlings in both unlogged and logged forest in Sabah, North Borneo, during a non-mast year. We test the following three hypotheses:
— seed and seedling survival will increase with distance from the nearest fruiting tree (distance dependence).
— seed and seedling survival will decrease with increased conspecific density (negative density dependence).
— the relationship between survival and distance and density will be stronger in the unlogged forest than in logged forest.
2. Methods
(a). Study site
The study was conducted around the Danum Valley Field Centre in Sabah, Malaysian Borneo (4°58′ N, 118°48′ E). Large areas of both logged and unlogged forest lie close to the field station. The Danum Valley Conservation Area (DVCA) contains 43 800 ha of unlogged, mostly lowland dipterocarp forest. The Yayasan Sabah Forest Management Area (YSFMA) is a logging concession of almost 1 million ha.
Logging in the YSFMA began in the mid-1970s and is ongoing. The areas included in this study were logged between 1970 and 1988. Timber was extracted using a combination of high lead on steep slopes and traditional tractor-based methods in other areas. Most of the healthy stems greater than 60 cm diameter at breast height (d.b.h.) were removed. Typical extraction rates were about 70 m3 ha−1 although higher rates have been reported (see [5] for further site information).
(b). Study species
Parashorea malaanonan is among the commonest species in the YSFMA and more widely in Sabah [5]. Permanent plots in the DVCA contain about 18.6 P. malaanonan stems ha−1 (greater than 10 cm girth at breast height) [34]. Most of the forest in the YSFMA is classified as P. malaanonan (type A) forest, a category that includes much of the natural vegetation in the upper Segama region and coastal areas of Eastern Sabah [5]. Parashorea malaanonan is commercially harvested and its timber is classed as White Serraya Light Hardwood (timber density 0.52 g cm−1 [32,35]). It is a relatively fast-growing dipterocarp and its seedlings perform better in gaps, and therefore it is considered relatively light demanding for a dipterocarp [36]. However, dipterocarps in general are very shade-tolerant and overall P. malaanonan has high survival [21,36] and growth rates [37] in understorey conditions.
Parashorea malaanonan appears to fruit more often than other dipterocarps at the study site, and successfully set seed in 1996, 2000 and 2004 (the year of this study, [29]). The winged seeds are dispersed by wind or gyration and mostly fall under the parent tree's canopy. Several vertebrates and invertebrates have been observed attacking seeds and seedlings of P. malaanonan, including bearded pigs Sus babatus (R. Bagchi 2004, personal observation), rodents [38] and insect seed predators and herbivores [36,39,40].
(c). Site selection
In August 2004, fruiting P. malaanonan trees were located in the unlogged forest by searching along trails around the field station. The logged forest was surveyed from the logging roads. Ten trees were identified for the study in both forest types. Trees were considered suitable if they had a large fruit crop, were greater than 30 m away from roads and greater than 100 m from other fruiting trees included in the study. A 30 m transect was established starting at the base of each selected tree. The direction of this transect was constrained to avoid trails and other fruiting P. malaanonan trees but otherwise chosen at random (i.e. a restricted random design; see electronic supplementary material, figure S1).
(d). Seedfall traps
Traps were used to establish seedfall rates soon after the first seedfall was observed. Traps were deployed at 2, 10, 20 and 30 m along the transects. Each 1 × 1 m trap was constructed by suspending a piece of plastic mesh above the ground with string attached to suitable trees. Dipterocarp seeds are dispersed by gyration and generally land close to the parent tree [41–43]. Our experience is that few seeds disperse further than 30 m and this is supported by the data presented here (figure 1). Seeds were collected from the traps in both forests every 4 days between 23 September and 30 November 2004. In addition, at each census, we collected five seeds (if possible) from the ground at each distance interval, but avoided the immediate vicinity of the traps and plots. These seeds would have been exposed to both pre-dispersal and post-dispersal insect predators. The collected seeds (trap and ground) were brought back to the laboratory in order to rear out insect seed predators (see §2g).
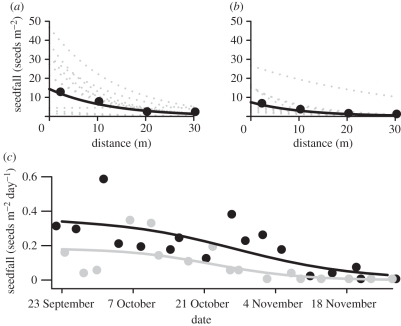
Figure 1.
The relationship between P. malaanonan seedfall and distance from the nearest fruiting tree in (a) unlogged and (b) logged forest. Points are the mean number of seeds falling into 1 m2 seed traps located at 2, 10, 20 and 30 m from 10 trees in each forest type. Solid lines represent the number of seeds predicted to fall at each distance by the model fitted to the data. Dotted lines represent the predictions for each of the 10 trees in each forest type. (c) The majority of seeds fell in the first six weeks of the study. Points are the mean number of seeds that fell in seed traps placed 2 m away from each tree and lines are the model predictions. Black line, unlogged; grey line, logged.
(e). Non-manipulated plots
A 1 × 1 m plot was established 2 m to the right of each seed trap. Seeds found in the plot were tagged with small numbered flags pinned to their wings. The presence and status of tagged seeds were checked at the same time as the seedfall traps, and new seeds were tagged and recorded. The status of each seed was recorded as potentially viable (intact seeds with no visible signs of fungal attack or insect exit holes), dead (decomposing or empty), germinated, fungus-infected, insect-predated (with exit holes), consumed by vertebrates (partially consumed seed remaining), seedling browsed by vertebrates or removed from the plot (presumed dead). Some of the removed seeds were possibly secondarily dispersed, but previous work on dipterocarp seed predation suggests that most are consumed [25,38]. Note that these categories were not exclusive. The plots were monitored until 30 November 2004.
(f). Density manipulation plots
Parashorea malaanonan seeds were collected from the vicinity of additional fruiting trees. To manipulate density and distance independently, we established two further 1 × 1 m plots at distances of 2 and 30 m from each focal tree, adjacent to the existing traps, on 9 October 2004. At each distance we assigned plots to high- or low-density treatments at random. We placed 25 (high density) or four (low density) tagged seeds on a regular grid in the plots. The high-density treatment (25 seeds m−2) corresponds to the highest density we found in the naturally dispersed seed plots. The low-density treatment (4 seeds m−2) is at the low end of seed densities observed naturally and provides some within-plot replication. All naturally dispersed seeds were removed throughout the experiment. These plots were censused until 30 November 2004, at the same time as the traps and non-manipulated plots. Seeds were assigned to the same categories as those in the non-manipulated plots (see earlier text).
(g). Insect rearing
Seeds collected from the ground and traps in different censuses were stored separately for rearing insects, but we pooled the seeds from each distance. Any visible signs of insect predation were recorded and then seeds were placed in ventilated rearing boxes lined with damp tissue paper. Seeds were examined every 3 days, and date of germination or emergence of seed predators or parasitoids recorded. Lepidopteran predators were pinned on emergence and dried. Emerged weevils and parasitoids were stored in 90 per cent ethanol in a freezer. After four months, all seeds were dissected, predation recorded and any larvae or adults still inside the seeds stored in alcohol. Specimens were mounted and identified at the Natural History Museum (London) and the Oxford University Museum of Natural History. Insects were classed as pre-dispersal or post-dispersal predators on the basis of the literature [44–46].
(h). Statistical analysis
We used generalized linear mixed-effects models (GLMMs) [47,48] for all analyses. Seedfall was modelled as a function of forest type and distance from the nearest fruiting tree, assuming a Poisson error distribution. The intercept and effect of distance were allowed to vary between trees as normally distributed random effects (random intercept and slope model).
Initial analyses suggested only small differences in survival between seeds in the manipulated and non-manipulated plots, so data were combined for analyses. Seeds recorded as dead, removed or eaten by vertebrates were categorized as ‘dead’, while seeds in other categories were regarded as survivors. Note that seeds in the ‘insect-predated’ and ‘fungus-infected’ categories were not initially counted as dead, but if the seed was determined to be dead on a subsequent visit, these agents were considered responsible for their death. For each plot, the number of seeds that died or survived during each census interval was recorded.
We modelled seed and seedling survival using GLMMs, assuming a binomial error distribution. We examined the effects of forest type, distance to the nearest fruiting tree, conspecific density at the start of the census interval and the two- and three-way interactions between these predictors. Total seedfall at each tree over the study was included as a measure of medium-scale seed density. Intercept terms for each census were included in the model as normally distributed, random effects. This allows the overall survival rate to change over time (for example, if older seeds or seedlings are less vulnerable), without making assumptions about the form of this relationship, similar to Cox proportional hazard models [49]. Preliminary analyses suggested that the relationship between survival and time differed considerably between the two forest types; so we included an interaction between census and forest type as a random effect. Intercept terms for trees and plots were included as normally varying random effects. The relationship between survival and time was also allowed to vary between plots as a random effect (random intercept and slope model).
The probability of predation by insects of seeds kept in the laboratory was modelled as a function of forest type and the total seed crop at each tree using a GLMM with a binomial error distribution. In a separate model, we examined the effect of distance from the nearest fruiting conspecific, forest type and their interaction using only the seeds collected from the forest floor. Predation of seeds collected in the traps will be dominated by pre-dispersal predation, and testing the effect of distance on predation of these seeds may therefore be inappropriate. In both models, separate intercepts for trees were modelled as normally distributed random effects.
There is much debate about how to appropriately test hypotheses using GLMMs [47,48]. We used the methodology recommended by Gelman & Hill [47] to construct 95% confidence intervals (CIs) for parameter estimates. We resampled 1000 times from the posterior distribution of the parameter estimates, and calculated the 2.5 and 97.5 per cent quantiles. An approximate, two-tailed, p-value was estimated as
where x is the number of samples greater than 0. We present these approximate p-values and the parameter estimates on the scale of the linear predictor with their 95% CI. Analyses were executed in R 2.11.1 [50] using the add-on packages lme4 0.999375-34 and arm 1.3-05. The R-code used to fit the models is available in the electronic supplementary material.
3. Results
(a). Seedfall
Overall, 353 seeds were collected from seed traps in the unlogged forest and 174 seeds in the logged forest (figure 1a,b, respectively). The difference was marginally non-significant (parameter estimate for the effect of logged forest 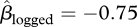 , 95% CI = −1.61–0.09, p = 0.076), and there was substantial variation between trees within forest types. The number of seeds in traps declined sharply with distance from focal trees, consistent with local seed dispersal (figure 1,
, 95% CI = −1.61–0.09, p = 0.076), and there was substantial variation between trees within forest types. The number of seeds in traps declined sharply with distance from focal trees, consistent with local seed dispersal (figure 1, 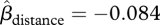 , 95% CI = −0.11 to −0.06, p < 0.001). Seedfall was heavy during the first few weeks of the study and began to decline after the first month of the study. Almost all seeds fell before the end of the sixth week (figure 1c).
, 95% CI = −0.11 to −0.06, p < 0.001). Seedfall was heavy during the first few weeks of the study and began to decline after the first month of the study. Almost all seeds fell before the end of the sixth week (figure 1c).
(b). Seed survival
Over two months, 332 seeds were naturally dispersed into the non-manipulated plots (217, 5.4 seeds m−2, in unlogged forest, 115, 2.9 seeds m−2, in logged forest). A further 1160 seeds were added to the 80 density manipulation plots (evenly distributed between the two forest types). No seeds fell into 23 of the non-manipulated plots, so the analyses are based on the remaining 57 non-manipulated and 80 manipulated plots.
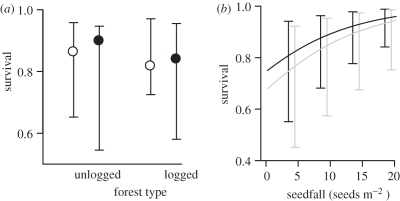
Of these seeds, 304 (38% of the non-manipulated and experimental seeds combined) survived to the end of the experiment in the unlogged forest compared with 117 (17%) in the logged forest. However, this difference was not significant (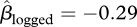 , 95% CI = −2.13–1.50, p = 0.746) because of the considerable variation in seed survival among trees (figure 2a). Only seven seeds (0.2 seeds m−2) remained in the non-manipulated plots in logged forest at the end of the study compared with 75 in the unlogged forest (1.9 seeds m−2). Total seedfall at a tree had a slight positive effect on seed survival (
, 95% CI = −2.13–1.50, p = 0.746) because of the considerable variation in seed survival among trees (figure 2a). Only seven seeds (0.2 seeds m−2) remained in the non-manipulated plots in logged forest at the end of the study compared with 75 in the unlogged forest (1.9 seeds m−2). Total seedfall at a tree had a slight positive effect on seed survival (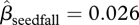 , 95% CI = −0.002–0.053, p = 0.072, figure 2b). There was a strong negative relationship between small-scale density and survival in both forest types (
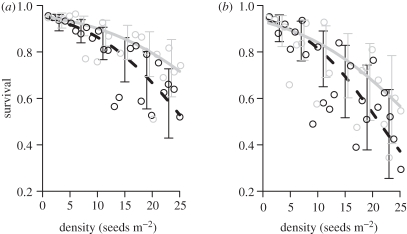
, 95% CI = −0.002–0.053, p = 0.072, figure 2b). There was a strong negative relationship between small-scale density and survival in both forest types (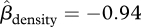 , 95% CI = −1.31 to −0.55, p < 0.001, figure 3). The strength of this relationship was unaffected by forest type (
, 95% CI = −1.31 to −0.55, p < 0.001, figure 3). The strength of this relationship was unaffected by forest type (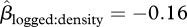 , 95% CI = −0.67–0.39, p = 0.574). Distance from the nearest fruiting tree did not affect seed survival significantly, and the interaction between distance and density was also non-significant (figure 2).
, 95% CI = −0.67–0.39, p = 0.574). Distance from the nearest fruiting tree did not affect seed survival significantly, and the interaction between distance and density was also non-significant (figure 2).
Figure 2.
(a) Survival of P. malaanonan seeds and seedlings in both unlogged and logged forest. Seed and seedling survival were not significantly affected by either forest type or distance from the nearest fruiting trees. Open circles, 2 m; filled circles, 30 m. (b) The effect of seed production at each tree on seed and seedling survival. Data are the mean survival rates (±s.e.), taking into account the variation between plots and focal trees. Black line, unlogged; grey line, logged.
Figure 3.
Survival of P. malaanonan seedlings declined with conspecific seed and seedling density in both (a) unlogged and (b) logged forest. Distance from nearest fruiting tree did not affect survival. Points are the observed proportion of seeds that survived through 4 day census intervals in 1 m2 plots at each density at 2 and 30 m from the focal trees. Lines represent the expectations of the model fitted to the data and error bars represent the standard errors. Black dashed line, 2 m; grey solid line, 30 m.
(c). Causes of mortality
Most seed mortality appeared to be due to vertebrate seed predators, with seeds either missing or found partially eaten in the vicinity of the plots (figure 4). A large proportion of individuals also died soon after germinating and subsequently being browsed by mammals. Insects and fungi contributed little to the overall mortality between them. This pattern was similar in both forest types (figure 4).
Figure 4.
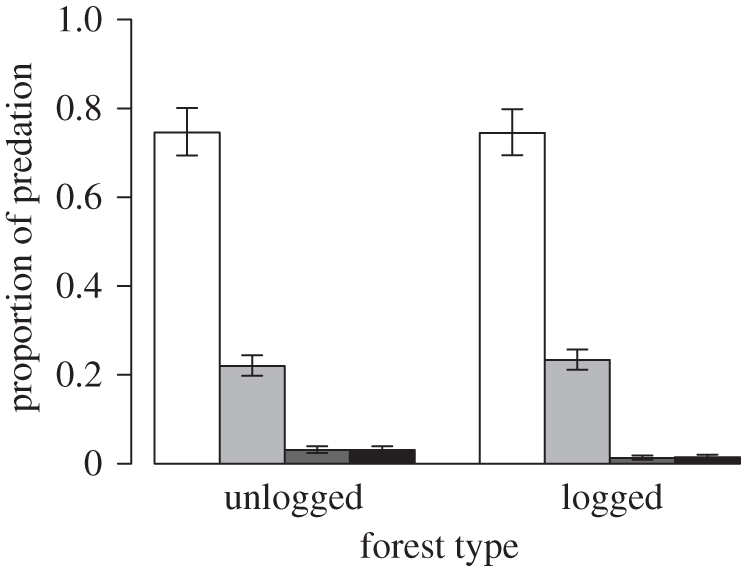
The causes of mortality of P. malaanonan seeds and seedlings in unlogged and logged forest. Data are the mean (±s.e.) proportion of deaths in which the mortality agent was implicated. Note that more than one agent could contribute to death; so the proportions can add up to greater than 1. Open bars, vertebrate predators; light grey bars, vertebrate browsers; dark grey bars, fungi; black bars, insects.
(d). Insect predation
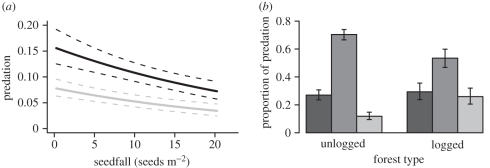
A total of 2337 seeds were collected from the traps (566 seeds) and the ground (1771 seeds) and brought back to the laboratory. Insects (moths, weevils or parasitoids) were reared from 210 (9%) of these seeds. A higher proportion of seeds in the unlogged forest were predated (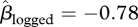 , 95% CI = −1.16 to − 0.34, p < 0.001, figure 5a). Predation was reduced at trees with large seed crops (
, 95% CI = −1.16 to − 0.34, p < 0.001, figure 5a). Predation was reduced at trees with large seed crops (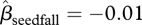 , 95% CI = −0.019 to −0.002, p = 0.012, figure 5a). There was no effect of distance on predation by insects of seeds collected from the forest floor.
, 95% CI = −0.019 to −0.002, p = 0.012, figure 5a). There was no effect of distance on predation by insects of seeds collected from the forest floor.
Figure 5.
(a) Insect predation on P. malaanonan seeds collected in traps or from the forest floor nearby was higher in logged forest than unlogged forest and at trees that produced fewer seeds. Lines are the predictions of the models fitted to the data (±s.e.). Black line, unlogged; grey line, logged. (b) The proportion of predated seeds attacked by different predators. Bars represent the mean (±s.e.) proportion of seed predators within three different categories and the proportion of predators that had been parasitized. Dark grey bars, moths; mid-grey bars, weevils; light grey bars, parasitoids.
The most important insect seed predators of P. malaanonan seeds were weevils of the Anthribidae and Curculionidae (figure 5b; electronic supplementary material, table S1). A micro-moth from the Tortricidae (probably Andrioplecta shoreae Komai; K. Tuck 2005, personal communication) was also an important pre-dispersal seed predator, accounting for all but two moths that emerged (see electronic supplementary material, table S1). When the seeds were dissected at the end of the experiment, several seeds contained dead larvae of weevils from the families Curculionidae (probably Alcidodes sp.) and Anthribidae (probably Araecerus sp.). However, no adult Alcidodes were successfully reared from the seeds, although a few adult Araecerus were. Parasitoids were reared from 18 per cent of predated seeds and were primarily of the families Braconidae and Ichneumonidae (figure 5b; electronic supplementary material, table S1). We assumed that seeds with parasitoids must have been attacked by seed predators first, and these were therefore counted as predated.
There were 41 seeds from the traps that had insect exit holes on collection, but no insects emerged from 22 of them. It is possible that these seeds had been predated by weevils of the family Nanophyidae, such as Nanophyes species, which often leave the seed before it falls from the tree [51].
4. Discussion
(a). Effects of logging on the Janzen–Connell mechanism
We found strong negative effects of small-scale (1 m2) conspecific density on the survival of P. malaanonan seeds and germinating seedlings in both unlogged and logged tropical forest. The strength of this density dependence was independent of logging history. This suggests either that logging has not affected seed-predator-mediated density dependence or this process has recovered within 15–35 years after logging.
Similar work in the Neotropics has generally found strong effects of human disturbance on density dependence of tree survival [30–32]. It is likely that this disparity with our data is due to differences in the type of disturbance to which the forests were subjected. While logging operations in the YSFMA extract large volumes of timber, hunting pressure on wildlife is relatively low [5]. The Neotropical studies compared forests subjected to intense hunting and missing key seed predators to relatively undisturbed ones. Indeed, hunting was identified as the major cause of the reduced density dependence in each case [30–32]. Furthermore, the Dipterocarpaceae are mainly abiotically dispersed [42] in contrast to many Neotropical species that will have lost dispersers as well as seed predators. Because our data are the first from South East Asia to compare density-dependent effects between unlogged and logged forests, it would be premature to conclude that hunting pressure, rather than regional differences, explains the differences between our results and those of previous studies. However, it provides the most likely explanation. The vertebrate faunas in many forests in the region suffer severely from hunting [3], so data comparable to those reported here could be collected from other South-East Asian forests.
While high conspecific density at very small scales increased mortality, seed predation in the field and by insects reared in the laboratory was lower at trees with large seed crops. Such positive effects of seed density are unsurprising because both pre-dispersal insect [13] and post-dispersal vertebrate [52] seed predators are likely to be satiated by large amounts of seeds. One explanation for the different response of post-dispersal predators to density at small and large scales is that while large seed crops eventually satiate them, they concentrate their foraging in areas of the seed shadow with the largest seed density. Seed predation by both insects and vertebrates was independent of proximity to the nearest fruiting adult, possibly because the disadvantages of being close to fruiting trees are offset by the ability of large amounts of seeds to satiate seed predators locally. The independence of insect seed predation to distance might reflect that a large proportion of the reared insects were pre-dispersal seed predators, even in the seeds collected from the forest floor.
While the strength of density dependence was very similar in unlogged and logged forests, seedfall and survival rates were less so. Mean seedfall and survival were both twice as high in the unlogged forest as the logged forest. Although the differences between forest types in both seedfall and survival were non-significant, this reflects the large amount of variation between focal trees within forest types rather than similar averages. Logging, inevitably, reduces the number of adults of timber species and this may explain reduced seed production in the logged forest. Increasing the distance of dipterocarps to the nearest flowering conspecific reduces cross-pollination and subsequently decreases seed set [25,53,54]. Logging has been observed to reduce seed production in previous work [53,54]. The seed crop in logged forest may therefore be reduced at the scale of the individual tree by high proportions of unpollinated and self-pollinated flowers, and at the landscape scale by a decrease in the number of adult trees. Combined with the trend for survival to be highest at trees with large seed crops, this led nearly all surviving seeds to be in unlogged forest. Only seven seeds remained in the non-manipulated plots in logged forest at the end of the study compared with 75 seeds in the unlogged forest. Thus, natural regeneration in the logged forest was practically non-existent. It should be noted that even in the unlogged forest, seedling densities were very low at the end of the experiment (less than 2 seed m−2), and may therefore make little contribution to the recruitment of this species.
One caveat applies to all our comparisons between unlogged and logged forest. While one of this study's strengths is that the forest types were relatively similar in composition prior to logging [5], we considered only one area each of unlogged and logged forest. Although the replicate trees within each forest type were far from each other (greater than 100 m), and may be considered independent samples, the conclusions of this study apply to the forests around the DVCA and YSFMA. Furthermore, we considered only one species. Parashorea malaanonan is very abundant and is a relatively fast-growing dipterocarp, making it an atypical species. Rarer species may be better able to escape from their natural enemies in space, and therefore show stronger distance and density dependence. Further studies at other paired areas of unlogged and logged forest, and with additional species, will be necessary to establish if the patterns discovered here apply to unlogged and logged forests in general.
(b). Causes of mortality
Vertebrates caused the vast majority of the observed seed and germinating seedling mortality in the field. It has been suggested that vertebrates are unlikely to cause such density dependence because they tend to be mobile generalists ([55], but see [56]). The mobility of these seed predators did not prevent them from generating strong negative density dependence in P. malaanonan survival. Because we did not consider heterospecifics here, we cannot determine if mortality increased with conspecific density or just density in general. For the Janzen–Connell mechanism to maintain species richness, increased density of a particular species must increase predation of heterospecifics less than conspecifics [57]. It is quite possible that seeds of other species would not benefit from a rare species advantage if they had dispersed into plots with high densities of P. malaanonan. Other work at this site, however, suggests that small vertebrates prefer conspecific dipterocarp seeds to heterospecific dipterocarp seeds, but large vertebrates did not discriminate between them [58].
Our field data probably underestimate insect predation of P. malaanonan. A proportion of insect-predated seeds would have been removed from the plots (assuming vertebrates did not discriminate against them). Such seeds would have been scored as vertebrate-predated, causing us to underestimate the role of invertebrates. While we examined seeds for signs of insect emergence holes, these can be easily missed in the field, especially because insects often emerge from between the wings of dipterocarp seeds. These wings are formed from the calyx, and P. malaanonan has two long (9–16 cm) and three short (6–10 cm) wings [59]. The area between the wings is very rough, and emergence holes there might be overlooked. Emergence rates from seeds kept in the laboratory provide a more realistic estimate of invertebrate attack. In the absence of vertebrate predators, insects attacked about 9 per cent of seeds, low in comparison with other studies from the region [25,51]. However, even our laboratory data will probably underestimate insect predation. About 54 per cent of the seeds we collected showed evidence of insect predation but did not produce any insects, and it is likely that seed predators left a proportion of these seeds before we collected them. Some weevils in the Nanophyidae have been recorded to emerge prior to seed dispersal [51]. Furthermore, much of the insect-related mortality of dipterocarp seeds occurs early on in fruit development, leading to abortion. These early losses can be substantial [51], but we only started collecting seeds once they were mature, and therefore almost certainly underestimated the impact of insects.
(c). Mast fruiting and the Janzen–Connell mechanism
It is generally thought that practically no dipterocarp recruitment occurs in South East Asian forests outside community-wide mast-fruiting events [27,28]. However in this study, 35 per cent of seeds in the unlogged forest and 17 per cent in logged forest survived until the end of two months. About 8 per cent of the original seedlings in the non-manipulated plots (18 individuals) were still alive in the unlogged forest sites 10 months later, equivalent to 0.45 seedlings m−2 (R. Bagchi 2005, unpublished data). Therefore, contrary to the general consensus, at least some dipterocarps recruited in a non-mast year, albeit in small numbers. It is of course very possible that these individuals will die before they reach maturity, or that their recruitment makes a negligible contribution to the population dynamics of P. malaanonan. While mortality rates are very high during the seed and early seedling stage described here, the seedlings that survived the duration of this study will probably have to survive for several decades in order to reach the canopy [42]. Without comparable data from a mast year, it is difficult to determine how the density of survivors in this study compares, but it is likely to be much lower than expected after a mast fruiting.
Maycock et al. [25] similarly reported recruitment outside major fruiting events of the congeneric Parashorea tomentella at Sepilok Forest Reserve, another lowland forest in Sabah. However, Maycock et al. [25] also reported negligible survival of other dipterocarp species in the same fruiting event. One possible explanation could be that P. malaanonan seeds are unpalatable. However, various seed predators (vertebrates and invertebrates) have been recorded attacking P. malaanonan seeds and seedlings [38,40], so this seems unlikely. Maycock et al. [25] suggested that P. tormentella satiated seed predators because a high proportion of trees fruited over a large area. Parashorea species are extremely common in both Danum and Sepilok, and this may partly explain its success outside community-wide mast events. However, data from very abundant species at other sites suggest no such patterns. Two very abundant dipterocarp species, Shorea lamellata and Shorea quadrinervis, both failed to recruit after producing seeds in non-mast years [27,60].
5. Conclusion
In this study, seeds and seedlings produced during a non-mast year were predated by vertebrate predators and, to a lesser extent, insects. This predation was negatively density dependent at small spatial scales in both forest types, suggesting species diversity of logged forests will return to pre-logging levels more rapidly than might have been expected otherwise. However, survival increased with density at a larger spatial scale, probably because predators were satiated. Seed production was much lower in logged forest, and combined with the positive effects of large-scale density on survival, this resulted in recruitment being almost completely concentrated in unlogged forest. The failure of P. malaanonan to recruit in logged forest raises concerns about the ability of some tree species to recover from logging. If similar patterns were observed during mast years, this would have serious implications for the viability of logged forests in the region. Determining whether this is the case should be considered a research priority.
Acknowledgements
This work was funded through PhD studentships to R.B. and E.M.S. (Natural Environment Research Council, UK) and C.D.P. (Swiss National Science Foundation) and a CASE studentship to E.M.S. (Natural History Museum, London). We thank the Danum Valley Management Committee and the Economic Planning Unit of the Prime Minister's Department, Kuala Lumpur, for permission to conduct research in Malaysia. We thank Kevin Tuck (Natural History Museum, London) and Darren Mann (Oxford University Museum of Natural History) for help with the identification of insect specimens. Arthur Chung (Forest Research Centre, Sabah) and Glen Reynolds (Director, Royal Society SEARRP) provided invaluable support. We thank Johnny Larenus, Jamil Hanapi, Philip Ulok, Anna Gust and Alex Karolus for their field assistance and Miranda Davis and two anonymous referees for comments on the initial manuscript. This paper constitutes Publication Number A/576 of the Royal Society South East Asia Rainforest Research Programme.
References
- 1.Curran L. M., Trigg S. N., McDonald A. K., Astiani D., Hardiono Y. M., Siregar P., Caniago I., Kasischke E. 2004. Lowland forest loss in protected areas of Indonesian Borneo. Science 303, 1000–1003 10.1126/science.1091714 (doi:10.1126/science.1091714) [DOI] [PubMed] [Google Scholar]
- 2.Achard F., Eva H. D., Stibig H. J., Mayaux P., Gallego J., Richards T., Malingreau J.-P. 2002. Determination of deforestation rates of the world's humid tropical forests. Science 297, 999–1002 10.1126/science.1070656 (doi:10.1126/science.1070656) [DOI] [PubMed] [Google Scholar]
- 3.Sodhi N. S., Koh L. P., Brook B. W., Ng P. K. L. 2004. Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 19, 654–660 10.1016/j.tree.2004.09.006 (doi:10.1016/j.tree.2004.09.006) [DOI] [PubMed] [Google Scholar]
- 4.McMorrow J., Talip M. A. 2001. Decline of forest area in Sabah, Malaysia: relationship to state policies, land code and land capability. Global Environ. Chang. 11, 217–230 10.1016/S0959-3780(00)00059-5 (doi:10.1016/S0959-3780(00)00059-5) [DOI] [Google Scholar]
- 5.Marsh C. W., Greer A. G. 1992. Forest-land use in Sabah, Malaysia: an introduction to Danum Valley. Phil. Trans. R. Soc. Lond. B 335, 331–339 10.1098/rstb.1992.0025 (doi:10.1098/rstb.1992.0025) [DOI] [Google Scholar]
- 6.Wright S. J., Muller-Landau H. C. 2006. The future of tropical forest species. Biotropica 38, 287–301 10.1111/j.1744-7429.2006.00154.x (doi:10.1111/j.1744-7429.2006.00154.x) [DOI] [Google Scholar]
- 7.Chazdon R. L., Peres C. A., Dent D., Sheil D., Lugo A. E., Lamb D., Stork N. E., Miller S. E. 2009. The potential for species conservation in tropical secondary forests. Conserv. Biol. 23, 1406–1417 10.1111/j.1523-1739.2009.01338.x (doi:10.1111/j.1523-1739.2009.01338.x) [DOI] [PubMed] [Google Scholar]
- 8.Dent D. H., Wright S. J. 2009. The future of tropical species in secondary forests: a quantitative review. Biol. Conserv. 142, 2833–2843 10.1016/j.biocon.2009.05.035 (doi:10.1016/j.biocon.2009.05.035) [DOI] [Google Scholar]
- 9.Foody G. M., Cutler M. E. J. 2003. Tree biodiversity in protected and logged Bornean tropical rain forests and its measurement by satellite remote sensing. J. Biogeogr. 30, 1053–1066 10.1046/j.1365-2699.2003.00887.x (doi:10.1046/j.1365-2699.2003.00887.x) [DOI] [Google Scholar]
- 10.Bischoff W., Newbery D. A., Lingenfelder M., Schnaeckel R., Petol G. H., Madani L., Ridsdale C. 2005. Secondary succession and dipterocarp recruitment in Bornean rain forest after logging. Forest Ecol. Manage. 218, 174–192 10.1016/j.foreco.2005.07.009 (doi:10.1016/j.foreco.2005.07.009) [DOI] [Google Scholar]
- 11.Lugo A. E. 2009. The emerging era of novel tropical forests. Biotropica 41, 589–591 10.1111/j.1744-7429.2009.00550.x (doi:10.1111/j.1744-7429.2009.00550.x) [DOI] [Google Scholar]
- 12.Berry N. J., et al. 2010. The high value of logged tropical forests: lessons from northern Borneo. Biodivers. Conserv. 19, 985–997 10.1007/s10531-010-9779-z (doi:10.1007/s10531-010-9779-z) [DOI] [Google Scholar]
- 13.Lewis O. T., Gripenberg S. 2008. Insect seed predators and environmental change. J. Appl. Ecol. 45, 1593–1599 10.1111/j.1365-2664.2008.01575.x (doi:10.1111/j.1365-2664.2008.01575.x) [DOI] [Google Scholar]
- 14.Lewis O. T. 2009. Biodiversity change and ecosystem function in tropical forests. Basic Appl. Ecol. 10, 97–102 10.1016/j.baae.2008.08.010 (doi:10.1016/j.baae.2008.08.010) [DOI] [Google Scholar]
- 15.Wright S. J. 2002. Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130, 1–14 [DOI] [PubMed] [Google Scholar]
- 16.Novotny V., Drozd P., Miller S. E., Kulfan M., Janda M., Basset Y., Weiblen G. D. 2006. Why are there so many species of herbivorous insects in tropical rainforests? Science 313, 1115–1118 10.1126/science.1129237 (doi:10.1126/science.1129237) [DOI] [PubMed] [Google Scholar]
- 17.Lewinsohn T., Roslin T. 2008. Four ways toward tropical herbivore megadiversity. Ecol. Lett. 11, 398–416 10.1111/j.1461-0248.2008.01155.x (doi:10.1111/j.1461-0248.2008.01155.x) [DOI] [PubMed] [Google Scholar]
- 18.Janzen D. H. 1970. Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528 10.1086/282687 (doi:10.1086/282687) [DOI] [Google Scholar]
- 19.Connell J. H. 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In Dynamics of numbers in populations (eds den Boer P. J., Gradwell G. R.), pp. 298–312 Wageningen, The Netherlands: PUDOC [Google Scholar]
- 20.Carson W. P., Anderson J. T., Leigh E. G., Schnitzer S. A. 2008. Challenges associated with testing and falsifying the Janzen–Connell hypothesis: a review and critique. In Tropical forest community ecology (eds Carson W. P., Schnitzer S. A.), pp. 210–241 Oxford, UK: Blackwell's [Google Scholar]
- 21.Bagchi R., Press M. C., Scholes J. D. 2010. Evolutionary history and distance dependence control survival of dipterocarp seedlings. Ecol. Lett. 13, 51–59 10.1111/j.1461-0248.2009.01397.x (doi:10.1111/j.1461-0248.2009.01397.x) [DOI] [PubMed] [Google Scholar]
- 22.Webb C. O., Peart D. R. 1999. Seedling density dependence promotes coexistence of Bornean rain forest trees. Ecology 80, 2006–2017 10.1890/0012-9658(1999)080[2006:SDDPCO]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[2006:SDDPCO]2.0.CO;2) [DOI] [Google Scholar]
- 23.Blundell A. G., Peart D. R. 2004. Density-dependent population dynamics of a dominant rain forest canopy tree. Ecology 85, 704–715 10.1890/01-4101 (doi:10.1890/01-4101) [DOI] [Google Scholar]
- 24.Itoh A., Yamakura T., Ogino K., Lee H. S. 1995. Survivorship and growth of seedlings of 4 dipterocarp species in a tropical rainforest of Sarawak, East Malaysia. Ecol. Res. 10, 327–338 10.1007/BF02347859 (doi:10.1007/BF02347859) [DOI] [Google Scholar]
- 25.Maycock C. R., Thewlis R. N., Ghazoul J., Nilus R., Burslem D. F. R. P. 2005. Reproduction of dipterocarps during low intensity masting events in a Bornean rain forest. J. Veg. Sci. 16, 635–646 10.1111/j.1654-1103.2005.tb02406.x (doi:10.1111/j.1654-1103.2005.tb02406.x) [DOI] [Google Scholar]
- 26.Curran L. M., Caniago I., Paoli G. D., Astianti D., Kusneti M., Leighton M., Nirarita C. E., Haeruman H. 1999. Impact of El Nino and logging on canopy tree recruitment in Borneo. Science 286, 2184–2188 10.1126/science.286.5447.2184 (doi:10.1126/science.286.5447.2184) [DOI] [PubMed] [Google Scholar]
- 27.Curran L. M., Leighton M. 2000. Vertebrate responses to spatiotemporal variation in seed production of mast-fruiting Dipterocarpaceae. Ecol. Monogr. 70, 101–128 10.1890/0012-9615(2000)070[0101:VRTSVI]2.0.CO;2 (doi:10.1890/0012-9615(2000)070[0101:VRTSVI]2.0.CO;2) [DOI] [Google Scholar]
- 28.Janzen D. H. 1974. Tropical blackwater rivers, animals and mast fruiting by the Dipterocarpaceae. Biotropica 6, 69–103 10.2307/2989823 (doi:10.2307/2989823) [DOI] [Google Scholar]
- 29.Bagchi R. 2006. Factors influencing the diversity of trees in southeast Asian rain forests. PhD thesis, University of Sheffield, Sheffield, UK [Google Scholar]
- 30.Wright S. J., Zeballos H., Dominguez I., Gallardo M. M., Moreno M. C., Ibanez R. 2000. Poachers alter mammal abundance, seed dispersal, and seed predation in a neotropical forest. Conserv. Biol. 14, 227–239 10.1046/j.1523-1739.2000.98333.x (doi:10.1046/j.1523-1739.2000.98333.x) [DOI] [Google Scholar]
- 31.Wyatt J. L., Silman M. R. 2004. Distance-dependence in two Amazonian palms: effects of spatial and temporal variation in seed predator communities. Oecologia 140, 26–35 10.1007/s00442-004-1554-y (doi:10.1007/s00442-004-1554-y) [DOI] [PubMed] [Google Scholar]
- 32.Dirzo R., Miranda A. 1991. Altered patterns of herbivory and diversity in the forest understorey: a case study of the possible consequences of contemporary defaunation. In Plant–animal interactions: evolutionary ecology in tropical and temperate regions (eds Price P. W., Lewinsohn T. M., Fernandes G. W., Benson W. W.), pp. 273–287 New York, NY: John Wiley and Sons [Google Scholar]
- 33.Curran L. M., Webb C. O. 2000. Experimental tests of the spatiotemporal scale of seed predation in mast-fruiting Dipterocarpaceae. Ecol. Monogr. 70, 129–148 10.1890/0012-9615(2000)070[0129:ETOTSS]2.0.CO;2 (doi:10.1890/0012-9615(2000)070[0129:ETOTSS]2.0.CO;2) [DOI] [Google Scholar]
- 34.Stoll P., Newbery D. M. 2005. Evidence of species-specific neighborhood effects in the Dipterocarpaceae of a Bornean rain forest. Ecology 86, 3048–3062 10.1890/04-1540 (doi:10.1890/04-1540) [DOI] [PubMed] [Google Scholar]
- 35.Burgess P. F. 1966. Timbers of Sabah. Sandakan, Sabah: Forest Department of Malaysia [Google Scholar]
- 36.Bebber D. P., Brown N. D., Speight M. R. 2002. Drought and root herbivory in understorey Parashorea Kurz (Dipterocarpaceae) seedlings in Borneo. J. Trop. Ecol. 18, 795–804 10.1017/S0266467402002511 (doi:10.1017/S0266467402002511) [DOI] [Google Scholar]
- 37.Philipson C. D., et al. In press Light-based regeneration niches: evidence from 21 dipterocarp species using size-specific RGRs. Biotropica. [Google Scholar]
- 38.Wells K., Bagchi R. 2005. Eat in or take away—seed predation and removal by rats (Muridae) during a fruiting event in a dipterocarp rainforest. Raffles B Zool. 53, 125–130 [Google Scholar]
- 39.Whitmore T. C., Brown N. D. 1996. Dipterocarp seedling growth in rain forest canopy gaps during six and a half years. Phil. Tran. R. Soc. Lond. B 351, 1195–1203 10.1098/rstb.1996.0102 (doi:10.1098/rstb.1996.0102) [DOI] [Google Scholar]
- 40.Slade E. M. 2007. The effects of tropical forest management on biodiversity and ecosystem functioning. PhD thesis, University of Oxford, Oxford, UK [Google Scholar]
- 41.Osada N., Takeda H., Furukawa A., Awang M. 2001. Fruit dispersal of two dipterocarp species in a Malaysian rain forest. J. Trop. Ecol. 17, 911–917 10.1017/S0266467401001687 (doi:10.1017/S0266467401001687) [DOI] [Google Scholar]
- 42.Whitmore T. C. 1984. Tropical rainforests of the Far East, 2nd edn. Oxford, UK: Oxford University Press [Google Scholar]
- 43.Kettle C. J., Hollingsworth P. M., Burslem D. F. R. P., Maycock C. R., Khoo E., Ghazoul J. 2011. Determinants of fine-scale spatial genetic structure in three co-occurring rain forest canopy trees in Borneo. Perspect. Plant Ecol. Evol. Syst. 13, 47–56.(doi:10.1016/j.ppees.2010.11.002) [Google Scholar]
- 44.Chey V. K. 2002. Dipterocarp seed predators. Malaysian Nat. 55, 46–49 [Google Scholar]
- 45.Lyal C. H. C., Curran L. M. 2000. Seed-feeding beetles of the weevil tribe Mecysolobini (Insecta: Coleoptera: Curculionidae) developing in seeds of trees in the Dipterocarpaceae. J. Nat. Hist. 34, 1743–1847 10.1080/00222930050122165 (doi:10.1080/00222930050122165) [DOI] [Google Scholar]
- 46.Lyal C. H. C., Robinson G. D., Intachat J., Bupabanpot J., Curran L. M. 2011. The Dipterocarp insect seed-predator host database. See http://www.nhm.ac.uk/research-curation/research/projects/dipterocarps/seed-predation/index.html (accessed January 2011).
- 47.Gelman A., Hill J. 2007. Data analysis using regression and multilevel/hierarchical models. New York, NY: Cambridge University Press [Google Scholar]
- 48.Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H. H., White J.-S. S. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 49.Egli P., Schmid B. 2001. The analysis of complex leaf survival data. Basic Appl. Ecol. 2, 223–231 10.1078/1439-1791-00048 (doi:10.1078/1439-1791-00048) [DOI] [Google Scholar]
- 50.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 51.Toy R. J., Marshall A. G., Pong T. Y. 1992. Fruiting phenology and the survival of insect fruit predators—a case-study from the South-East Asian Dipterocarpaceae. Phil. Trans. R. Soc. Lond. B 335, 417–423 10.1098/rstb.1992.0033 (doi:10.1098/rstb.1992.0033) [DOI] [Google Scholar]
- 52.Schupp E. W. 1992. The Janzen–Connell model for tropical tree diversity—population implications and the importance of spatial scale. Am. Nat. 140, 526–530 10.1086/285426 (doi:10.1086/285426) [DOI] [PubMed] [Google Scholar]
- 53.Murawski D. A., Gunatilleke I., Bawa K. S. 1994. The effects of selective logging on inbreeding in Shorea megistophylla (Dipterocarpaceae) from Sri Lanka. Conserv. Biol. 8, 997–1002 10.1046/j.1523-1739.1994.08040997.x (doi:10.1046/j.1523-1739.1994.08040997.x) [DOI] [Google Scholar]
- 54.Ghazoul J., Liston K. A., Boyle T. J. B. 1998. Disturbance-induced density-dependent seed set in Shorea siamensis (Dipterocarpaceae), a tropical forest tree. J. Ecol. 86, 462–473 10.1046/j.1365-2745.1998.00270.x (doi:10.1046/j.1365-2745.1998.00270.x) [DOI] [Google Scholar]
- 55.Hammond D. S., Brown V. K. 1998. Disturbance, phenology and life-history characteristics: factors influencing distance/density-dependent attack on tropical seeds and seedlings. In Dynamics of tropical communities (eds Newbery D. M., Prins H. H. T., Brown N. D.), pp. 51–78 Oxford, UK: Blackwell's [Google Scholar]
- 56.Paine C. E. T., Beck H. 2007. Seed predation by neotropical rain forest mammals increases diversity in seedling recruitment. Ecology 88, 3076–3087 10.1890/06-1835.1 (doi:10.1890/06-1835.1) [DOI] [PubMed] [Google Scholar]
- 57.Hille R., Lambers J., Clark J. S., Beckage B. 2002. Density-dependent mortality and the latitudinal gradient in species diversity. Nature 417, 732–735 10.1038/nature00809 (doi:10.1038/nature00809) [DOI] [PubMed] [Google Scholar]
- 58.Hautier Y., Saner P., Philipson P., Bagchi R., Ong R. C., Hector A. 2010. Effects of seed predators of different body size on seed mortality in Bornean logged forest. PLoS ONE 5, e11651. 10.1371/journal.pone.0011651 (doi:10.1371/journal.pone.0011651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newman M. F., Burgess P. F., Whitmore T. C. 1999. Malesian Dipterocarps. Foresters CD-ROM Manual. Edinburgh, UK: Royal Botanic Garden, Edinburgh [Google Scholar]
- 60.Blundell A. G., Peart D. R. 2004. Seedling recruitment failure following dipterocarp mast fruiting. J. Trop. Ecol. 20, 229–231 10.1017/S0266467403001123 (doi:10.1017/S0266467403001123) [DOI] [Google Scholar]