Abstract
Stable carbon isotope (δ13C) series were developed from analysis of sequential radial wood increments from AD 1850 to AD 2009 for four mature primary rainforest trees from the Danum and Imbak areas of Sabah, Malaysia. The aseasonal equatorial climate meant that conventional dendrochronology was not possible as the tree species investigated do not exhibit clear annual rings or dateable growth bands. Chronology was established using radiocarbon dating to model age–growth relationships and date the carbon isotopic series from which the intrinsic water-use efficiency (IWUE) was calculated. The two Eusideroxylon zwageri trees from Imbak yielded ages of their pith/central wood (±1 sigma) of 670 ± 40 and 759 ± 40 years old; the less dense Shorea johorensis and Shorea superba trees at Danum yielded ages of 240 ± 40 and 330 ± 40 years, respectively. All trees studied exhibit an increase in the IWUE since AD 1960. This reflects, in part, a response of the forest to increasing atmospheric carbon dioxide concentration. Unlike studies of some northern European trees, no clear plateau in this response was observed. A change in the IWUE implies an associated modification of the local carbon and/or hydrological cycles. To resolve these uncertainties, a shift in emphasis away from high-resolution studies towards long, well-replicated time series is proposed to develop the environmental data essential for model evaluation. Identification of old (greater than 700 years) ringless trees demonstrates their potential in assessing the impacts of climatic and atmospheric change. It also shows the scientific and applied value of a conservation policy that ensures the survival of primary forest containing particularly old trees (as in Imbak Canyon and Danum).
Keywords: Borneo, stable isotope, dendrochronology, REDD, water-use efficiency, carbon
1. Introduction
(a). Tropical trees as recorders of environmental change
Decision-making in conservation management is often constrained by unacceptably high levels of uncertainty surrounding estimates of forest response to environmental pressures [1]. An ability to quantify more accurately the age-structure of a forest and the flux of carbon and water through it may help reduce these uncertainties and provide a method with which to evaluate and improve Earth systems models. One area of growing interest is the effect that carbon dioxide concentrations, which have increased during the industrial era from approximately 285 ppm in AD 1850 to greater than 392 ppm in AD 2010 [2–4], may have on the forest biosphere and its ability to cycle water and nutrients [5].
Plants regulate gas exchange and moisture loss through subtle changes in leaf morphology and metabolic processes that are sensitive to environmental change and which may be investigated through analysis of the stable isotopic composition of the plant [4]. Such changes influence the fluxes of both carbon dioxide and water, thus influencing the climatic system; this process is termed physiological forcing [6,7].
One of the key physiological forcings is changing stomatal conductance. Plant stomata evolved to internalize gas exchange and limit moisture loss, and there is a strong tendency to maintain the balance between the concentration of carbon dioxide outside (ca) and inside (ci) the leaf. As the concentration of carbon dioxide in the atmosphere increases, plants can potentially maintain the ratio ci/ca by reducing stomatal conductance and thus reducing moisture loss per unit of carbon gained (increasing their water-use efficiency) [8]. Climate model-based studies have shown that reductions in plant stomatal conductance can impact both directly and indirectly upon regional climate, soil moisture, stream-runoff and productivity [9–11]. Uncertainty in the magnitude and significance of this physiological forcing arises, because most studies on the response of stomatal conductance to rising CO2 are extrapolated from short-term, leaf ‘exposure’ studies [11], which cannot take account of acclimation that may alter the stomatal response over longer timescales [6,12] or of potential thresholds to response, which may already have been reached [13,14].
One way to reduce these uncertainties is to investigate naturally occurring archives of plant physiological change, such as the wood of trees. This approach has been applied successfully in high-latitude regions where clear annual rings allow well-replicated, absolutely dated chronologies to be constructed, providing annually resolved archives of information on past environmental change [4]. Across much of the tropics, in areas where seasonality is absent/weakly expressed, trees generally do not produce clear annual rings and so chronologies cannot be constructed by dendrochronology alone. Here that problem is overcome by using radiocarbon dating to estimate tree age and growth rate, providing the first record of changing plant response over time, using ringless trees from the lowland dipterocarp rainforest in Sabah, Malaysia.
(b). Carbon isotope fractionation and intrinsic water-use efficiency
Farquhar et al. [15] proposed a model to describe carbon isotopic fractionation in plants that relates, in its simple form, the carbon isotopic composition of the resulting photosynthates to (i) the isotopic composition and concentration of the source carbon dioxide, (ii) plant regulation of CO2 entering the leaf (stomatal conductance) and (iii) the rate at which it is fixed by photosynthesis (assimilation rate; equation 1.1). This may be modified to express carbon isotope fractionation in terms of isotopic discrimination (Δ) (equation 1.2).
| 1.1 |
and
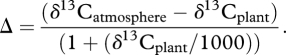 |
1.2 |
As the conductance of the leaf to water vapour is 1.6 times that of CO2, it is possible to express discrimination in terms of net assimilation (A) and conductance to water vapour (gw), which is termed the intrinsic water-use efficiency (IWUE; equation 1.3) [8].
| 1.3 |
where:
δ13Cplant, stable carbon isotopic composition of the photosynthate;
δ13Catmosphere, stable carbon isotopic composition of the atmospheric CO2 used in photosynthesis;
a, fractionation due to diffusion of CO2 through the stomata (4.4‰);
b, fractionation by ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) enzyme during carboxylation (approx. 27‰);
ci, inter-cellular concentration of CO2 (CO2 concentration internal to the leaf);
ca, concentration of atmospheric CO2 (CO2 concentration external to the leaf);
Δ, discrimination;
A, rate of CO2 assimilation by the plant;
gw, the conductance of the boundary layer and stomatal pores to the diffusion of water (H2O).
Several studies have demonstrated that trees can modify their IWUE through changes in the number, size and density of the stomata as levels of atmospheric carbon dioxide increase [16–19]. Kürschner [20] demonstrated that deciduous European trees responded through a general increase in IWUE, but at CO2 concentrations of about 340 ppm appeared to reach a threshold beyond which their response levelled off. Other tree-ring-based studies [21–24] have reported broadly similar results. Waterhouse et al. [14] concluded that the magnitude and the timing of the diminished response were likely to be both site- and species-specific, and argued that a reassessment of estimated global carbon budgets and the modelling of feedbacks might now be required.
In a study of IWUE in ringed trees from the somewhat seasonal location of Aripuaña, Brazil (10°09′ S, 59°26′ W), Hietz et al. [25] sampled carbon isotope ratios in homogenized blocks of dated tree-rings of Cedrela odorata L. and Swietenia macrophylla King. Although the pooled samples included several very young trees, their results indicated that the IWUE in that part of Amazonia had increased by 34 per cent for C. odorata and 52 per cent for S. macrophylla between AD 1850 and AD 2000. As no continuous individual isotope time series were developed, however, it was not possible to determine whether or not the trees had reached a plateau in their response.
The IWUE differs from instantaneous water-use efficiency (instWUE) as the former does not depend on the vapour pressure gradient between the leaf and the surrounding air. The IWUE has the advantage over instWUE in that it allows assessment of the role of biological components in determining water–carbon exchange relationships independently of changing atmospheric conditions [14,26]. The IWUE, however, does not take into account the effects of external climate variability, respiration or mesophyll conductance [27], and without this additional information it is difficult to determine whether changes in the IWUE are driven by assimilation rate, gross conductance or a combination of both factors. Notwithstanding these limitations, tree ring IWUE provides a fundamental insight into plant water-use, both currently and retrospectively.
(c). Developing chronology from ringless tropical trees
Trees growing in temperate seasonal climates usually display well-defined tree-rings, the widths of which are partly controlled by environmental factors, such as temperature and moisture availability. This allows cross-dating (synchronization) between trees and between sites, which is the basis for dendrochronology (tree-ring dating). Although some of the earliest tree-ring studies were conducted in the seasonally wet tropics [28,29], it has been generally assumed that regions where climate does not exhibit a strong seasonal control on tree growth are unsuitable for dendrochronology [30]. Although some studies in seasonal tropical environments have demonstrated the presence of coherent growth bands in tropical timbers and thereby provide new opportunities for tree-ring research [29,31,32], over large areas of the tropics trees do not form regular growth bands. Ringless tropical trees may, nevertheless, provide a rich palaeoecological archive because they can be very long-lived [33], and assuming that wood formation proceeds without protracted dormancy, the stable isotopes of carbon, oxygen and non-exchangeable hydrogen fixed in the wood cells could provide a continuous record of changes in environment and in physiological response.
Dendrochronology and dendroclimatology rely upon replication to establish robust chronology and to quantify signal strength. Attempts to produce annually resolved chronologies for ringless trees, using high-resolution analyses [34–39], have resulted in the identification of rhythmic patterns in wood chemistry that may be annual in nature. However, to be useful in dendrochronology, high-resolution isotope series need to be replicated. To analyse five 50 cm long cores at 10 µm resolution would require a minimum of 250 000 isotopic determinations, which constitutes an unrealistic analytical commitment. A shift in emphasis and analytical approach is therefore required, away from the high-frequency analysis of single ringless trees towards larger scale better replicated studies. Radiocarbon dating can provide an estimate of tree age and if dating uncertainty can be addressed and adequately quantified then ringless tropical archives could provide information on lower frequency environmental changes. This approach is essentially no different from that used to investigate peat cores and lake or ocean sediments. By accepting some dating uncertainty, and focusing on changes in environment at greater than annual timescales, it may prove possible to provide the information so urgently required by the modelling community for such regions. This study will assess the potential of such an approach through analysis of the IWUE developed using ringless trees from the aseasonal lowland dipterocarp rainforests of Sabah, Malaysia.
2. Material and methods
(a). Site description, sample collection and analysis
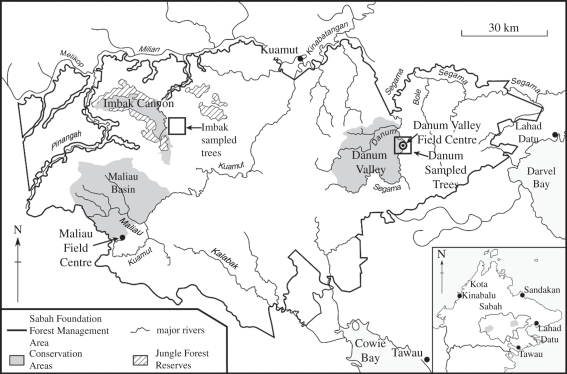
Four trees were sampled from two lowland rainforest locations in Sabah, Malaysian Borneo. At Danum Valley Conservation Area (04°57′ N, 117°48′ E; figure 1), one of the largest remaining regions of pristine lowland dipterocarp forest, disc samples of Shorea superba and Shorea johorensis trees were collected from sites about 300 m altitude on gently sloping terrain. The S. superba (a mature tree outside the conservation area) and the S. johorensis (a canopy tree within a long-term forest ecology plot inside the conservation area) had both been blown over immediately prior to sampling during storms in March 2001 (S. superba) and June 2009 (S. johorensis). The climate of Danum is equatorial with a mean annual rainfall (1985–2009) of 2873 mm. There is no dry season and monthly rainfall means range from 313 mm in January to 159 mm in April. During El Niño/Southern Oscillation events, however, dry periods with two to four successive months with less than 100 mm of rain occur, as in 1992 and 1998. The geology is a heterogeneous melange of Miocene siltstones, sandstones, cherts, spillites and tuffs and soils are mainly FAO haplic alisols (Alh), comprising 38–57% sand, 20–35% silt and 13–27% clay [40,41]. At Imbak (05°53′ N, 117°05′ E) two samples of Eusideroxylon zwageri (local name Belian) trees were collected in an area of moderate relief (200–400 m altitude) and sandstone geology east of Imbak Canyon Conservation Area. Limited meteorological data suggest a climate broadly similar to that of Danum Valley, although somewhat wetter (over 3000 mm per annum). Borneo represents an important study region because its everwet climate is affected by the Indo-Australian Monsoon System and is strongly affected by El Niño/Southern Oscillation events. Few high-resolution terrestrial palaeoenvironmental records exist for this region.
Figure 1.
Location map of Sabah, Borneo, identifying the study site locations at Danum Valley and Imbak Canyon.
The four trees sampled were mature canopy trees and apparently healthy (with intact wood across the entire discs) prior to felling. With the exception of the S. johorensis, samples were from near-ground stem avoiding buttresses and rotten timber. All tree sections exhibited asymmetrical growth, nevertheless it was possible to select a single radius from each sample for both radiometric dating and stable isotopic analysis to avoid disturbance/reaction tissues, and represented, as far as was possible, the natural annular growth form.
Samples were prepared for radiocarbon dating using a scalpel and microdrill to obtain the greater than 0.6 g of wood powder required for cellulose extraction. Care was taken to ensure that the wood powder did not char, which could cause isotopic fractionation. Each dated radius was sub-sampled for stable carbon isotope analysis. Samples were divided into contiguous 1 mm sections and further subdivided into thin slivers using a scalpel prior to isolation of cellulose [42–44]. The S. superba prepared independently by Robertson et al. [45] was also cut radially and α-cellulose prepared. Owing to its greater size, for practical reasons, sequential wholewood rather than cellulose samples were analysed for the S. johorensis. These two components preserve similar signals, but an adjustment of 1 per mille is made to the wholewood carbon isotope ratios prior to calculation of IWUE to account for the isotopic offset between wholewood and cellulose [46].
Between 300 and 350 µg of dry homogenized α-cellulose or dry wood powder were weighed into tin-foil capsules and combusted quantitatively to carbon dioxide over chromium(III) oxide at 1000°C using a Sercon GSL Elemental Analyser interfaced with a 20/20 isotope ratio mass spectrometer. Results are expressed using the delta (‘δ’) notation relative to the Vienna PeeDee Belemnite (VPDB) standard [4]. Precision, based on replicate analyses of an in-house standard, is typically 0.1 per mille (σn−1, n = 10). The S. superba series developed by Robertson et al. [45] was analysed in a similar manner.
(b). Chronology and age–growth modelling
A simple age–growth model was produced by fitting a negative exponential curve between radiocarbon dating control points. The radiocarbon method can provide several possible dates for a sample depending on the points at which it intercepts the radiocarbon calibration curve, but the use of multiple dates can reduce significantly this uncertainty [45]. The modelled δ13C series were annualized to facilitate calculation of the IWUE by interpolation and/or combination depending on the temporal resolution of the growth model. The temporal uncertainty in the radiocarbon dates (±40 years, 1 sigma) was expressed in terms of δ13C and IWUE as one standard deviation in the range of values falling within the dating uncertainty for each annualized point. While this approach simplifies a relatively complex and non-normally distributed error structure, it does permit a conservative calculation of uncertainty point-for-point in each series.
3. Results and discussion
(a). Chronology
The radiocarbon dating results (±1 sigma) of near-pith wood of the two E. zwageri trees (AD 1330 and AD 1250) suggest ages of 670 ± 40 and 759 ± 40 years, respectively, which given the relatively small radial growth sampled (18 and 29 cm, respectively) indicates very slow growth rates averaging ca 0.27 and 0.38 mm yr−1. By contrast, the ages of the S. superba and S. johorensis trees are much lower at 330 ± 40 and 240 ± 40 years, respectively, indicating faster mean growth rates for these dipterocarps (sampled radii 44 and 65 cm, respectively). In the Imbak Canyon Conservation Area, many E. zwageri specimens exceed 2 m in diameter, suggesting ages of at least 1500 years. The dates support the findings of Kurokawa et al. [33] who also identified the long-lived and slow-growing nature of this species through analysis of stump and remnant samples in neighbouring Sarawak. Such long-lived trees offer potential for developing millennial length records in the everwet tropics for palaeoclimatic research. They highlight also the considerable age of the trees growing in the primary forest and thus demonstrate how rapidly felling can remove the carbon fixed into an ecosystem over many centuries. It is important that such slow-cycling is taken into account when developing conservation and management strategies [47].
(b). Stable carbon isotopes and intrinsic water-use efficiency
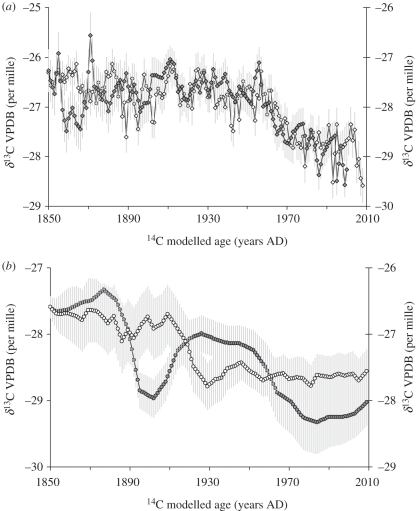
The annualized stable carbon isotope data (figure 2a,b) all exhibit a characteristic declining trend, which can be related to parallel changes in the carbon isotopic composition of atmospheric carbon dioxide following the onset of global industrialization (ca AD 1850). At Danum Valley in particular, the S. superba and S. johorensis display an encouraging degree of coherence. The E. zwageri records show slightly lower coherence, and a smoother trend reflecting the lower temporal resolution of each millimetre increment analysed (approx. 2 and 5 years growth, respectively).
Figure 2.
Graphs showing the raw stable isotope series for the four trees studied at (a) Danum (S. superba and S. johorensis) and (b) Imbak (E. zwageri). Filled diamonds, S. superba; open diamonds, S. johorensis; filled circles, E. zwageri (Tree T6B2); open circles, E. zwageri (Tree T5B1). Grey error bars represent the uncertainty in age–growth model (1 sigma ≈ ±40 years) expressed as the running standard deviation of tree response over a 40 year period.
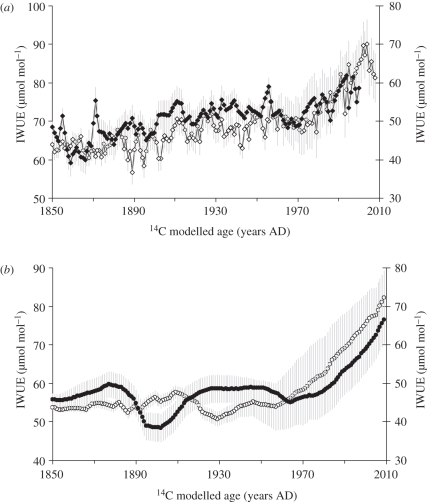
Calculation of the IWUE for each of the trees (figure 3a,b) also reveals a common response over time, although a slight decline in the IWUE of the naturally felled S. johorensis immediately prior to death is observed, which may indicate a change in the health of the tree making it more susceptible to windthrow or the onset of a plateau in IWUE. At both sites, absolute values are in a range similar to those reported for tropical and boreal species [6,14,21–25]. All series remain relatively stable until ca AD 1960–1970, after which the trees improve their IWUE coincident with a change in the rate of increase of atmospheric CO2 concentration. Increasing IWUE can result from either (or a combination of) an increase in assimilation rate (which affects carbon sequestration) or a decrease in stomatal conductance (which more directly influences the hydrological cycle and climate system), and it is currently not possible to determine which factor dominates the response observed here as the trends observed may be driven by either changing carbon dioxide concentrations or a change in climate, or both.
Figure 3.
Intrinsic water-use efficiency (IWUE) calculated for each tree and plotted against time for (a) Danum and (b) Imbak. Filled diamonds, S. superba; open diamonds, S. johorensis; filled circles, E. zwageri (Tree T6B2); open circles, E. zwageri (Tree T5B1). Grey error bars represent the uncertainty in age–growth model (1 sigma ≈ ±40 years) expressed as the running standard deviation of tree response over a 40 year period. Note: a 1 per mille adjustment to account for the wholewood/cellulose isotopic difference has been made to the S. johorensis series prior to calculation of IWUE.
Gagen et al. [6] were able to deconvolve the IWUE signal through paired analysis of tree-rings and sub-fossil leaves. They identified stomatal conductance as the dominant driver of changing IWUE at a boreal site in northern Fennoscandia. In the tropics, historic leaf material (albeit limited) available in herbaria and large tracts of forest and riverine peat deposits make the preservation and development of an equivalent archive of sub-fossil leaf material an exciting possibility and potential source of additional information on past forest response.
While there are few inland meteorological records available for this region, a record of relative humidity at the Danum site (AD 1985–to date) exhibits no trend in monthly relative humidity recorded at 08.00 h but a slight positive trend in data recorded at 14.00 h over the past 25 years. Through its effect on stomatal conductance, however, an increase in relative humidity should logically have the effect of reducing IWUE (assuming assimilation rate remains constant), so it is unlikely that humidity alone is driving the changes observed. Sunshine (photon flux) directly controls the rate of photosynthesis and the amount of carbon sequestered. At Danum, analysed records of sunshine are only available since AD 2009, so it is impossible to discern whether the increase in the IWUE is the result of increased photosynthetic rate. Several studies have reported an increase in wood formation in tropical trees during recent decades [47,48] and have related this to increased levels of atmospheric carbon dioxide. Such direct evidence of a possible CO2 fertilization effect on trees growing under natural environmental conditions worldwide remains, however, limited and difficult to detect [49–51]. It is not possible to determine from these data alone whether or not a similar increase in carbon sequestration and allocation in woody biomass are also occurring across Sabah.
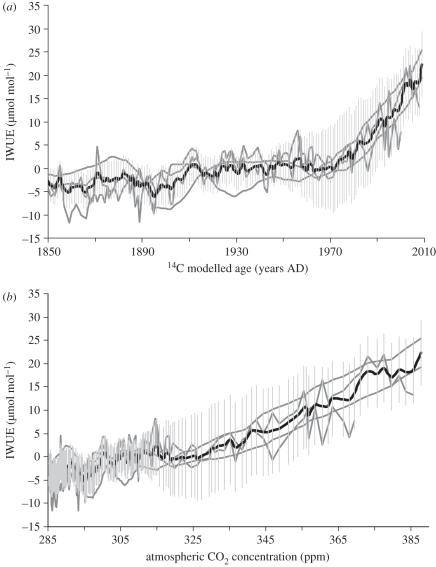
Figure 4 illustrates the individual series expressed as departures in IWUE from the mean of the four trees calculated for the common period (AD 1850–2000) and plotted against both time and atmospheric CO2 concentration. The magnitude of the mean response observed (29%) between AD 1850 and AD 2000 is broadly in line with values (23–48%) obtained from tree-based studies of the IWUE from high-latitude regions and only slightly less than that reported by Hietz et al. [25] for Brazilian trees. In contrast to Waterhouse et al. [14] and McCarroll et al. [52], there is no conclusive evidence for a sustained levelling-off in the response of the trees to increasing carbon dioxide concentrations at either Danum Valley or Imbak Canyon and it would appear, within the limitations of the approach, that at present trees are maintaining a stable level of ci/ca as levels of atmospheric CO2 increase. What is not known is whether and for how long this trend will continue in the future. These findings, therefore, provide a unique and timely motivation for integrating modelling and monitoring initiatives across the South East Asian rainforest to permit more accurate prediction, detection and attribution of future changes in water–plant–carbon interactions.
Figure 4.
Composite graphs showing the relationship between the individual tree IWUE series plotted against (a) time and (b) atmospheric CO2 concentration. All series are expressed as deviations from the mean calculated for the common period (AD 1850–2000). Thick grey lines represent the individual series, the thick black line represents the mean response (four trees) and the vertical error bars fitted to the mean (fine grey lines) represent the maximum annual uncertainty associated with the age–growth modelling of the four trees (1 sigma ≈ ±40 years).
4. Conclusions
(a). Conclusions, recommendations and future opportunities
This study has analysed the stable carbon isotopic composition of primary rainforest trees over the last 160 years for a region where aseasonal climate prevents the formation of annual growth bands and the application of conventional dendrochronological techniques. Radiocarbon dating was used to develop a timescale for dendrochemical analyses and to demonstrate how coherent (palaeo)environmental information can be successfully extracted from this archive.
Further to the development of high-resolution dendrochemical studies conducted in the tropics thus far, this study proposes an alternative, non-annual approach for extracting palaeoenvironmental data from ringless tropical trees. Although such an approach is also resource-intensive, this change of emphasis will facilitate development of the data urgently required for climate model evaluation in this region. A non-annual approach also permits increased replication and greater temporal coverage than is typically achieved using high-resolution methods alone. Further improvements can be made to the age–growth modelling component most easily through (i) the addition of further radiocarbon dates, (ii) incorporation of probabilistic (Bayesian or Monte Carlo) methods and (iii) combining the low-frequency signals with high-resolution elemental (Ca or stable C,H,O isotopic) analyses, where these are known to be temporally reliable.
Calculation of the IWUE for each of the trees demonstrated that at both sites the trees increased their IWUE by ca 29 per cent over the last 160 years (from a mean IWUE = 52 µmol mol−1 in AD 1851–1860 to 67 µmol mol−1 in AD 1991–2000). The change is similar in magnitude to that found in other climatic and vegetational zones and represents an active/plastic response of the trees to rising atmospheric CO2. Were the trees insensitive to rising CO2, one would expect to see the amount of CO2 within the leaves (ci) increase in parallel with levels outside the leaves (ca), so that ca − ci (and thus IWUE) would remain stable through time. Instead the trees appear to have maintained a near constant ci/ca ratio despite rising ambient levels of CO2, so that the increase in ci is less than the increase in ca. It is this ‘active response’ [52] that produces the ‘physiological forcing’ via a reduction in stomatal conductance, and therefore evapotranspiration, and/or an increase in carbon assimilation rate. A similar active response to increases in atmospheric CO2, reflected in increasing IWUE, has been identified in several tree-ring isotope chronologies from temperate areas, but in most cases there is evidence that the limits to plasticity have already been reached [14,20,21,52]. The change from an active to a passive response to increasing CO2 has important implications for climatic and carbon-cycle modelling. As described above, the calculation of IWUE from tree-ring data has limitations, and although only based on four trees, these results confirm that there is no universal level of CO2 beyond which trees cannot continue to respond actively, but that the limits to plasticity are likely to be species- and site-specific. Nevertheless, it seems unlikely that there are no limits to the plasticity of tropical trees, as anthropogenic activities in the near future appear likely to drive atmospheric CO2 concentrations beyond those experienced during the evolution of most living tree species [52].
The E. zwageri trees sampled were by no means the largest growing at Imbak Canyon, yet radiocarbon dating revealed these to be remarkably long-lived (ca 700 years old). The presence of many living E. zwageri trees of well over twice the breast height diameter of the sampled trees in the Imbak Canyon Conservation Area implies that tree ages well in excess of this age are likely. The identification of such ancient trees in the South East Asian rainforest supports the vision of resource management organizations such as Yayasan Sabah in identifying and designating primary forest conservation areas. It highlights the timescales that must be considered when replanting and regenerating tropical forests and in the development of carbon-offsetting schemes (through the United Nations Collaborative Programme on Reducing Emissions from Deforestation and Forest Degradation in Developing Countries; REDD). The immense ecological value and fragility of these ancient ecosystems, their palaeoecological potential and value for monitoring the impacts of CO2 and climatic change emphasize the urgent need for their further conservation.
Acknowledgements
We thank Danum Valley Management Committee and the Economic Planning units of the Malaysian Federal and Sabah State Governments for granting research permission; the UK NERC NE/B501504, the Royal Society South East Asia Rainforest Research Programme, ‘Millennium’ 017008 and C3W for supporting this research; Jamil Hanapi, Mike Barnardos and Vani Annammala for their invaluable help in the field; Rhodri Griffiths, Marcus Hull, Anna Ratcliffe and Jonathan Woodman-Ralph for technical and field assistance, Jadda Suhaimi (Imbak) and David Newbery (Bern) for helpful comments on development of this research. This paper constitutes Publication Number A/583 of the Royal Society South East Asia Rainforest Research Programme.
References
- 1.IPCC 2007. Climate change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Avery K. B., Tignor M., Miller H. L.), Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.NOAA. 2010. Trends in atmospheric CO2. Earth System Research Laboratory, National Oceanographic and Atmospheric Administration, United States Department of Commerce. See ftp://ftp.cmdl.noaa.gov/ccg/co2/trends/co2_mm_mlo.txt. (accessed July 2010)
- 3.Francey R. J., Allison C. E., Etheridge D. M., Trudinger C. M., Enting I. G., Leuenberger M., Langenfelds R. L., Michel E., Steele L. P. 1999. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus 51B, 170–193 10.1034/j.1600-0889.1999.t01-1-00005.x (doi:10.1034/j.1600-0889.1999.t01-1-00005.x) [DOI] [Google Scholar]
- 4.McCarroll D., Loader N. J. 2004. Stable isotopes in tree rings. Quat. Sci. Rev. 23, 771–801 10.1016/j.quascirev.2003.06.017 (doi:10.1016/j.quascirev.2003.06.017) [DOI] [Google Scholar]
- 5.Malhi Y., Grace J. 2004. Tropical forests and atmospheric carbon dioxide. Tree 15, 332–337 [DOI] [PubMed] [Google Scholar]
- 6.Gagen M., Finsinger W., Wagner-Cremer F., McCarroll D., Loader N. J., Robertson I., Jalkanen R., Young G., Kirchhefer A. 2010. Evidence of changing intrinsic water use efficiency under rising atmospheric CO2 concentrations in boreal Fennoscandia from subfossil leaves and tree ring δ13C ratios. Global Change Biol. 17, 1064–1072 10.1111/j.1365-2486.2010.02273.x (doi:10.1111/j.1365-2486.2010.02273.x) [DOI] [Google Scholar]
- 7.Betts R. A., Cox P. M., Woodward F. I. 2000. Simulated responses of potential vegetation to doubled-CO2 climate change and feedbacks on near-surface temperature. Global Ecol. Biogeogr. 9, 171–180 10.1046/j.1365-2699.2000.00160.x (doi:10.1046/j.1365-2699.2000.00160.x) [DOI] [Google Scholar]
- 8.Osmond C. B., Björkman O., Anderson D. J. 1980. Physiological processes in plant ecology. Ecol. Stud. 36, 1–468 [Google Scholar]
- 9.Cox P. M., Betts R. A., Bunton C. B., Essery R. L. H., Rowntree P. R., Smith J. 1999. The impact of new land surface physics on the GCM simulation of climate and climate sensitivity. Clim. Dyn. 15, 183–203 10.1007/s003820050276 (doi:10.1007/s003820050276) [DOI] [Google Scholar]
- 10.Sellers P. J., et al. 1996. Comparison of radiative and physiological effects of doubled atmospheric CO2 on climate. Science 271, 1402–1406 10.1126/science.271.5254.1402 (doi:10.1126/science.271.5254.1402) [DOI] [Google Scholar]
- 11.Ainsworth E. A., Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising CO2 mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270 10.1111/j.1365-3040.2007.01641.x (doi:10.1111/j.1365-3040.2007.01641.x) [DOI] [PubMed] [Google Scholar]
- 12.National Research Council, Climate Research Committee, National Research Council 2003. Understanding climate change feedbacks. Washington, DC: The National Academies Press [Google Scholar]
- 13.Kürschner W. M., Wagner F., Visscher E. H., Visscher H. 1997. Predicting the response of leaf stomatal frequency to a future CO2-enriched atmosphere: constraints from historical observations. Geol. Rundsch. 86, 512–517 10.1007/s005310050158 (doi:10.1007/s005310050158) [DOI] [Google Scholar]
- 14.Waterhouse J. S., Switsur V. R., Barker A. C., Carter A. H. C., Hemming D. L., Loader N. J., Robertson I. 2004. Northern European trees show a progressively diminishing response to increasing atmospheric carbon dioxide concentrations. Quat. Sci. Rev 23, 803–810 10.1016/j.quascirev.2003.06.011 (doi:10.1016/j.quascirev.2003.06.011) [DOI] [Google Scholar]
- 15.Farquhar G. D., O'Leary M. H., Berry J. A. 1982. On the relationship between carbon dioxide discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 9, 121–137 10.1071/PP9820121 (doi:10.1071/PP9820121) [DOI] [Google Scholar]
- 16.Leuzinger S., Körner C. 2007. Water savings in mature deciduous forest trees under elevated CO2. Global Change Biol. 13, 2498–2508 10.1111/j.1365-2486.2007.01467.x (doi:10.1111/j.1365-2486.2007.01467.x) [DOI] [Google Scholar]
- 17.Marshall J. D., Monserud R. A. 1996. Homeostatic gas-exchange parameters inferred from C-13/C-12 in tree rings of conifers. Oecologia 105, 13–21 10.1007/BF00328786 (doi:10.1007/BF00328786) [DOI] [PubMed] [Google Scholar]
- 18.Woodward F. I., Kelly C. K. 1995. The influence of CO2 concentration on stomatal density. New Phytol. 131, 311–327 [Google Scholar]
- 19.Royer D. L. 2001. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palyn. 114, 1–28 10.1016/S0034-6667(00)00074-9 (doi:10.1016/S0034-6667(00)00074-9) [DOI] [PubMed] [Google Scholar]
- 20.Kürschner W. M. 1996. Leaf stomata as biosensors of palaeoatmospheric CO2levels. PhD thesis, LPP Contributions Series No. 5, 153 pp, Utrecht University, Utrecht, The Netherlands [Google Scholar]
- 21.Feng X. 1999. Trends in intrinsic water-use efficiency of natural trees for the past 100–200 years: a response to atmospheric CO2 concentrations. Geochim. Cosmochim. Acta 63, 1891–1903 10.1016/S0016-7037(99)00088-5 (doi:10.1016/S0016-7037(99)00088-5) [DOI] [Google Scholar]
- 22.Bert D., Leavitt S. W., Dupouey J.-L. 1997. Variations of wood δ13C and water-use efficiency of Abies alba during the last century. Ecology 78, 1588–1596 10.1890/0012-9658(1997)078[1588:VOWCAW]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[1588:VOWCAW]2.0.CO;2) [DOI] [Google Scholar]
- 23.Duquesnay A., Breda N., Stievenard M., Dupouey L. 1998. Changes of tree-ring δ13C and water-use efficiency of beech (Fagus sylvatica L.) in north-east France during the past century. Plant Cell Environ. 21, 565–572 10.1046/j.1365-3040.1998.00304.x (doi:10.1046/j.1365-3040.1998.00304.x) [DOI] [Google Scholar]
- 24.Saurer M., Siegwolf R. T. W., Schweingruber F. H. 2004. Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Global Change Biol. 10, 2109–2120 10.1111/j.1365-2486.2004.00869.x (doi:10.1111/j.1365-2486.2004.00869.x) [DOI] [Google Scholar]
- 25.Hietz P., Wanek W., Dünisch O. 2005. Long-term trends in cellulose δ13C and water-use efficiency of tropical Cedrela and Swietenia from Brazil. Tree Physiol. 25, 745–752 10.1093/treephys/25.6.745 (doi:10.1093/treephys/25.6.745) [DOI] [PubMed] [Google Scholar]
- 26.Ehleringer J. R. 1993. Carbon and water relations in desert plants: an isotopic perspective. In Stable isotopes and plant carbon–water relations (eds Ehleringer J. R., Hall J. R., Farquhar G. D.), pp. 155–172 San Diego, CA: Academic Press [Google Scholar]
- 27.Seibt U., Rajabi A., Griffiths H., Berry J. A. 2008. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia. 155, 441–454 10.1007/s00442-007-0932-7 (doi:10.1007/s00442-007-0932-7) [DOI] [PubMed] [Google Scholar]
- 28.van Bemmelen W. 1916. Drooftejaren op Java. Natuurk.Tijdschr. Ned. Indie 75, 157–179 [Google Scholar]
- 29.Worbes M. 2002. One hundred years of tree-ring research in the tropics–a brief history and an outlook to future challenges. Dendrochronologia 20, 217–231 10.1078/1125-7865-00018 (doi:10.1078/1125-7865-00018) [DOI] [Google Scholar]
- 30.Whitmore T. C. 1990. An introduction to tropical rain forests. Oxford, UK: Oxford University [Google Scholar]
- 31.Buckley B. M., Palakit K., Duangsathaporn K., Sanguantham P., Prasomsin P. 2007. Decadal scale droughts over northwestern Thailand over the past 448 years: links to the tropical Pacific and Indian Ocean sectors. Clim. Dyn. 29, 63–71 10.1007/s00382-007-0225-1 (doi:10.1007/s00382-007-0225-1) [DOI] [Google Scholar]
- 32.Sano M., Buckley B. M., Sweda T. 2009. Tree-ring based hydroclimate reconstruction over northern Vietnam from Fokienia hodginsii: eighteenth century mega-drought and tropical Pacific influence. Clim. Dyn. 33, 331–340 10.1007/s00382-008-0454-y (doi:10.1007/s00382-008-0454-y) [DOI] [Google Scholar]
- 33.Kurokawa H., Yoshida T., Nakamura T., Lai J., Nakashizuka T. 2003. The age of tropical rain-forest canopy species, Borneo ironwood (Eusideroxylon zwageri), determined by 14C dating. J. Trop. Ecol. 19, 1–7 10.1017/S0266467403003018 (doi:10.1017/S0266467403003018) [DOI] [Google Scholar]
- 34.Poussart P. M., Myeni S. C. B., Lanzirotti A. 2006. Tropical dendrochemistry: a novel approach to estimate age and growth from ringless trees. Geophys. Res. Lett. 33, L17711. 10.1029/2006GL026929 (doi:10.1029/2006GL026929) [DOI] [Google Scholar]
- 35.Helle G., Treydte K. S., Verheyden A. 2004. Tropical Swietenia macrophylla wood reveals a systematic recurring carbon isotope pattern. In TRACE Tree Rings in Archaeology, Climatology and Ecology. Proc. of the Dendrosymposium 2003, Utrecht, The Netherlands, vol. 2 (eds Jansma E., Bräuning A., Gärtner H., Schleser G. H.), pp. 107–109 Schriften des Forschungszentrums Jülich, Environment, Volume 44. Germany: Forschungszentrum Jülich GmbH 10.1016/j.gca.2008.11.041 (doi:10.1016/j.gca.2008.11.041) [DOI] [Google Scholar]
- 36.Evans M. N., Schrag D. P. 2004. A stable isotope-based approach to tropical dendroclimatology. Geochim. Cosmochim. Acta 68, 3295–3305 10.1016/j.gca.2004.01.0061353 (doi:10.1016/j.gca.2004.01.0061353) [DOI] [Google Scholar]
- 37.Poussart P., Schrag D. 2005. Seasonally resolved stable isotope chronologies from northern Thailand deciduous trees. Earth Planet. Sci. Lett. 235, 752–765 10.1016/j.epsl.2005.05.012 (doi:10.1016/j.epsl.2005.05.012) [DOI] [Google Scholar]
- 38.Poussart P., Evans M., Schrag D. 2004. Resolving seasonality in tropical trees: multi-decade, high-resolution oxygen and carbon isotope records from Indonesia and Thailand. Earth Planet. Sci. Lett. 218, 301–316 10.1016/S0012-821X(03)00638-1 (doi:10.1016/S0012-821X(03)00638-1) [DOI] [Google Scholar]
- 39.Anchukaitis K. J., Evans M. N., Wheelwright N. T., Schrag D. P. 2008. Stable isotope chronology and climate signal calibration in neotropical montane cloud forest trees. J. Geophys. Res. 113, G03030. 10.1029/2007JG000613 (doi:10.1029/2007JG000613) [DOI] [Google Scholar]
- 40.Leong K. M. 1974. The geology and mineral resources of the Upper Segama Valley and Darvey Bay area, Sabah, Malaysia, Memoir 41.(revised). Kuching: Government Printer [Google Scholar]
- 41.Chappell N. A., Ternan J. L., Bidin K. 1999. Correlation of physicochemical properties and sub-erosional landforms with aggregate stability variations in a tropical Ultisol disturbed by forestry operations. Soil Tillage Res. 50, 55–71 10.1016/S0167-1987(98)00196-2 (doi:10.1016/S0167-1987(98)00196-2) [DOI] [Google Scholar]
- 42.Loader N. J., Robertson I., Barker A. C., Switsur V. R., Waterhouse J. S. 1997. A modified method for the batch processing of small whole wood samples to a-cellulose. Chem. Geol. 136, 313–317 [Google Scholar]
- 43.Rinne K. T., Boettger T., Loader N. J., Robertson I., Switsur V. R., Waterhouse J. S. 2005. On the purification of alpha-cellulose from resinous wood for stable isotope (H, C and O) analysis. Chem. Geol. 222, 75–82 10.1016/j.chemgeo.2005.06.010 (doi:10.1016/j.chemgeo.2005.06.010) [DOI] [Google Scholar]
- 44.Loader N. J., et al. 2008. Multiple stable isotopes from oak trees in southwestern Scotland and the potential for stable isotope dendroclimatology in maritime climatic regions. Chem. Geol. 252, 62–71 10.1016/j.chemgeo.2008.01.006 (doi:10.1016/j.chemgeo.2008.01.006) [DOI] [Google Scholar]
- 45.Robertson I., Froyd C. A., Walsh R. P. D., Newbery D., Woodborne S., Ong R. C. 2004. The dating of dipterocarp tree rings: establishing a record of carbon cycling and climatic change in the tropics. J. Quat. Sci. 19, 657–664 10.1002/jqs.885 (doi:10.1002/jqs.885) [DOI] [Google Scholar]
- 46.Loader N. J., Robertson I., McCarroll D. 2003. Comparison of stable carbon isotope ratios in the whole wood cellulose and lignin of oak tree-rings. Palaeogeogr. Palaeoclimatol. Palaeoecol. 196, 395–407 10.1016/S0031-0182(03)00466-8 (doi:10.1016/S0031-0182(03)00466-8) [DOI] [Google Scholar]
- 47.Luyssaert S., Schulze E., Börner A., Knohl A., Hessenmöller D., Law B. E., Ciais P., Grace J. 2008. Old-growth forests as global carbon sinks. Nature 455, 213–215 10.1038/nature07276 (doi:10.1038/nature07276) [DOI] [PubMed] [Google Scholar]
- 48.Lewis S. L., et al. 2004. Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long-term plots. Phil. Trans. R. Soc. Lond. B 359, 421–436 10.1098/rstb.2003.1431 (doi:10.1098/rstb.2003.1431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graybill D. A., Idso S. B. 1983. Detecting the aerial fertilization effect of atmospheric CO2 enrichment in tree-ring chronologies. Global Biogeochem. Cycles 7, 81–95 [Google Scholar]
- 50.Koutavas A. 2008. Late 20th century growth acceleration in greek firs (Abies cephalonica) from Cephalonia Island, Greece: a CO2 fertilization effect? Dendrochronologia 26, 13–19 10.1016/j.dendro.2007.06.001 (doi:10.1016/j.dendro.2007.06.001) [DOI] [Google Scholar]
- 51.Jacoby G. C., D'Arrigo R. D. 1994. Tree rings, carbon dioxide, and climatic change. Proc. Natl. Acad. Sci. USA 94, 8350–8353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarroll D., et al. 2009. Correction of tree ring stable carbon isotope chronologies for changes in the carbon dioxide content of the atmosphere. Geochim. Cosmochim. Acta 73, 1539–1547 10.1016/j.gca.2008.11.041 (doi:10.1016/j.gca.2008.11.041) [DOI] [Google Scholar]