Abstract
Large areas of tropical forest now exist as remnants scattered across agricultural landscapes, and so understanding the impacts of forest fragmentation is important for biodiversity conservation. We examined species richness and nestedness among tropical forest remnants in birds (meta-analysis of published studies) and insects (field data for fruit-feeding Lepidoptera (butterflies and moths) and ants). Species–area relationships were evident in all four taxa, and avian and insect assemblages in remnants typically were nested subsets of those in larger areas. Avian carnivores and nectarivores and predatory ants were more nested than other guilds, implying that the sequential loss of species was more predictable in these groups, and that fragmentation alters the trophic organization of communities. For butterflies, the ordering of fragments to achieve maximum nestedness was by fragment area, suggesting that differences among fragments were driven mainly by extinction. In contrast for moths, maximum nestedness was achieved by ordering species by wing length; species with longer wings (implying better dispersal) were more likely to occur at all sites, including low diversity sites, suggesting that differences among fragments were driven more strongly by colonization. Although all four taxa exhibited high levels of nestedness, patterns of species turnover were also idiosyncratic, and thus even species-poor sites contributed to landscape-scale biodiversity, particularly for insects.
Keywords: community composition, guilds, ecosystem functioning, metapopulations
1. Introduction
Tropical forests are becoming increasingly fragmented by the widespread and rapid intensification of anthropogenic activities [1–4]. Formerly extensive tracks of continuous forests now exist as patchworks of isolated remnants scattered across inhospitable landscapes of non-forest habitats. This has resulted in the remaining forest patches supporting increasingly isolated populations of forest-dependent species [5]. Tropical rainforests support the majority of global biodiversity and contain large numbers of endemic species, and so understanding the impacts of forest fragmentation in these areas is crucial to the conservation of biodiversity [6–8].
There is a large literature examining the effects of tropical forest fragmentation on animals [1,9], including vertebrates (e.g. [10–14]) and insects [15–20]. Many previous studies have focused on describing changes in species richness following fragmentation, and have typically assessed the conservation value of forest remnants through the use of species–area relationships (SARs), which measure the rate at which species richness is lost as fragment size declines [21]. However, it is not clear how consistent SARs are within or among different taxa. Moreover, SARs may give a distorted view of the broader impacts of fragmentation on forest remnants because they provide no information on how faunal composition is altered by habitat fragmentation. Species losses may be offset by replacements from the surrounding matrix or elsewhere, so that SARs may underestimate changes in species composition following fragmentation, but it is unclear to what extent this might affect SARs in fragmented tropical forests.
An additional commonly observed property of biotas in fragmented habitats is that they are nested, such that species present at species-poor sites are subsets of those present at more species-rich sites [22,23]. Examination of the degree of nestedness has been used to infer the degree to which changes in species richness following fragmentation follow a predictable sequence [10,24–29]. However, few studies have evaluated whether different taxonomic groups follow the same patterns of nestedness or whether these patterns are generated by the same underlying mechanisms in each case [23].
Nestedness of species assemblages in forest fragments may arise as a result of four different processes (passive sampling, selective extinction, selective colonization and habitat nestedness [29]), which predict different patterns of nestedness among species and among fragments. Passive sampling could generate nestedness as a consequence of the fact that rare species are less likely to be sampled in a given area than common species, leading to the prediction that species with low average abundance will be a nested subset of those with high average abundance. Selective extinction predicts that in systems experiencing species loss or ‘relaxation’ post-fragmentation, area will be the main factor explaining species nestedness because species with large minimum-area requirements have greater extinction risk [30]. Selective colonization predicts that fragment isolation will create nested subsets of species through dispersal limitation [31,32]. Habitat nestedness considers the nestedness of species assemblages to arise as a result of their reliance on habitats that have a nested distribution [30]. Differences in the processes generating nestedness have important implications for conservation and can be used to direct management efforts [29], but are only poorly understood.
In addition to the characteristics of fragments, the traits of species may also provide useful information for assessing the importance of different processes in generating patterns of nestedness. For instance, if susceptibility to extinction is a strong determinant of nestedness, then traits linked to extinction proneness (e.g. geographical range size, habitat specificity [33,34]) may order species occurrence patterns. In contrast, if dispersal ability is a major driver, then traits linked to vagility (e.g. wing length [35,36]) may be more important in ordering species. However, this approach has received little attention [29].
Fragmentation of habitats has been shown to affect biotic interactions between species [37], such as herbivory [38–42] and seed predation [43]. There is also evidence that fragmentation can lead to trophic cascades [44–46], raising concerns that remnants of forest may not be viable in the longer term [47,48]. Nonetheless, small remnants may be important for promoting diversity in highly modified landscapes [49], and may also boost pollination success in surrounding agricultural crops (e.g. coffee [50,51]). Hence, patterns of nestedness among different trophic guilds may have important consequences for ecosystem functioning, but these have seldom been considered.
In this paper, we use information from published studies together with our own field data to examine how different ecological and functional groups of birds and insects respond to tropical forest fragmentation. There is an extensive literature on avian responses to fragmentation, as well as data on ecological traits (e.g. [14,52,53]), which we use to examine whether there is a consensus in SARs and patterns of nestedness among a wide range of tropical bird studies and among different feeding guilds. Most studies to date have focused on single taxa, but it is unclear the degree to which species and taxon responses are idiosyncratic or predictable [54,55]. Hence, we also analyse field data for butterflies, moths and ants from forest fragments in Sabah (Malaysian Borneo). The insect data were collected from many of the same forest fragments, allowing us a rare opportunity to evaluate the importance of different processes in generating SARs and patterns of nestedness among three insect taxa at the same locations.
2. Methods
(a). Avian responses to fragmentation
(i). Source data
We searched for suitable peer-reviewed studies examining avian responses to tropical forest fragmentation in online bibliographic databases (ISI Web of Science, EDINA Biosis); references within these were a source of further studies for consideration. Studies were included if they enumerated the species richness of each forest fragment studied, or provided data from which this could be obtained. Studies were included only if they provided data from three or more fragments. If fragments were compared at more than one distinct geographical location within a single published study, then data for each location were included separately such that each study provided more than one dataset for analysis. The area and isolation (distance from continuous forest) of fragments were taken from the authors' accounts or estimated from figures and maps using SigmaScan software (www.sigmaplot.com). We also recorded the surrounding landscape matrix as either cultivated, grassland, water or other (urban and mixed matrices).
(ii). Species richness within fragments
To evaluate the consistency in avian responses to tropical forest fragmentation, we first used linear regression to parameterize and determine the statistical significance of the SAR in each study. Methods for measuring species richness of fragments varied among the published studies and so, to ensure as far as possible that sampling effort was equal among fragments, we used individual-based rarefaction [56] to standardize sample sizes across fragments within each study. In some studies, species richness estimates for fragments were the result of historical inventories rather than discrete sampling regimes and these studies were excluded from the analysis. We then combined data across studies (N = 26) and used general linear modelling to relate the slopes (z) of the SARs to factors associated with extinction (range of fragment areas encompassed by study (i.e. max–min), and colonization (average isolation of fragments from continuous forest). We also controlled for possible confounding effects by including: habitat of the surrounding landscape matrix, time since fragmentation, duration and method of sampling (mist-net or observation), latitude, elevation and region (Neotropic, Africa or Asia) of study in the analysis. All predictor variables were included in the initial model, and the final model was obtained following serial deletion of least significant factors [57].
(iii). Nestedness and avian ecological traits
To examine the importance of different processes in generating patterns of nestedness, we first generated a species by site matrix for each study, indicating the presence or absence of each species within each fragment. We then used Binmatnest software [22] to reorder the matrix to achieve maximum nestedness among fragments and to compute a temperature index (T) scaled to vary between 0 (perfect nestedness, ‘cold’) and 100 (random order, ‘hot’). We chose this method over commonly used alternatives (e.g. the nestedness temperature calculator; [58]) because Binmatnest is a more robust test [29]. As recommended by the authors [22], we used null model 3 to examine whether the assemblages of birds in the different studies were significantly nested. Within each study, we then used Spearman correlations to examine whether or not the ranked order of fragments within the maximally packed matrix was related to fragment area. We next combined data across studies to examine how the degree of nestedness (temperature index, T) was related to variables linked to extinction, colonization or potential confounding effects (using general linear modelling with the same predictor variables as for the analysis of the slopes of SARs above). We based our analysis of nestedness on presence–absence rather than abundance data [59] because we were primarily interested in extinction and colonization processes rather than those generating variation in abundance within occupied sites.
To examine how the life-history traits of species influenced their degree of nestedness among fragments, we conducted separate analyses for subsets of species differing in body mass, geographical range size and feeding guild. Data for body mass were from [60] and data for species' geographical range sizes were from Birdlife International (www.birdlife.org). Species were classified into one of six feeding guilds (carnivore (including raptor, piscivore, faunivore, predator), frugivore, granivore, insectivore (including ant-follower, bark/foliage-gleaner, woodpecker), nectarivore, and omnivore (including scavenger, ‘miscellaneous’, and any species spanning two or more guilds)) according to their diet as reported by authors in the studies. For each study with suitable data, the nestedness of each feeding guild was quantified and compared with the nestedness of the total assemblage. For analyses of body mass and range size, assemblages were divided into two according to the median value, and nestedness was compared between the two groups.
(b). Insect responses to forest fragmentation
(i). Study sites
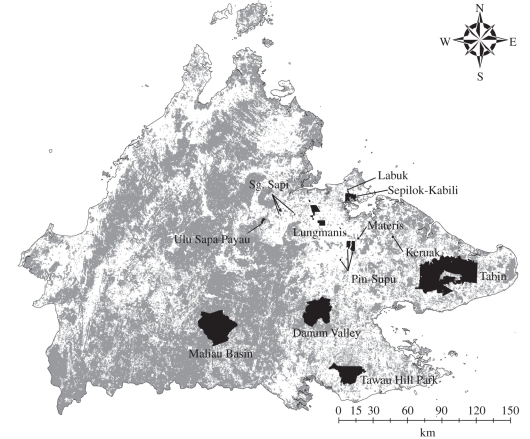
Leaf litter ants and fruit-feeding moths and butterflies were sampled at 13 sites in Sabah (Northern Borneo), comprising two widely separated ‘control’ sites within a single large area (approx. 1 million ha) of continuous forest (Danum Valley and Maliau Basin protected areas) and eight (Lepidoptera) or nine (ants) sites within forest remnants varying in size from 45 ha to approximately 120 000 ha (figure 1). Sample locations were all in lowland eastern Sabah, to reduce any residual effects of β-diversity prior to fragmentation. At the time of sampling, forest fragments were surrounded by an agricultural landscape containing mainly oil palm (Elaeis guineensis Jacq.) plantations, and had probably been isolated for approximately 50 years [61]. Isolation of each fragment, measured in terms of the minimum distance to continuous forest using a regional map of Sabah, varied from 6 to 69 km (figure 1).
Figure 1.
Map of Sabah showing locations of study sites (shaded black; NB some sites are very small). Sizes of forest fragments: Tabin, 122 540 ha; Tawau Hill Park, 91 590 ha; Sepilok-Kabili, 4290 ha; Lungmanis, 3390 ha; Ulu Sapa Payau, 720 ha; Keruak, 640 ha; Sungai Sapi C, 500 ha; Pin Supu, 218 ha; Materis, 225 ha; Labuk, 120 ha; Sungai Sapi A, 45 ha. Ants were sampled from all sites except Tawau Hill Park and Lungmanis; Lepidoptera were sampled from all sites except Materis and Sungai Sapi A. Areas of forest (light grey) and non-forest (white; mainly agriculture (oil palm)) are also shown.
(ii). Insect sampling
Obtaining complete inventories of species at each site was not possible and so, following previous studies [62], we used a standardized sampling technique to determine species richness in terms of the number of species in a uniform sampling area. We used standardized techniques to sample species along 2 km transects established at each study site. All samples were from locations more than 200 m within forest, to reduce edge effects [63]. Fruit-feeding moths (Families Noctuidae and Geometridae) and butterflies (Family Nymphalidae) were sampled with traps baited with rotting banana, hung at stations at 100 m intervals along each transect. This guild comprises about 75 per cent of all nymphalid butterflies recorded on Borneo [64,65] but a much smaller proportion of noctuid and geometrid moths [66–73]. Traps were suspended 2 m above ground level at each station and fresh banana was added to the trap each day to ensure a mixture of fresh and well-rotted bait [74]. Traps were emptied daily for 12 consecutive days, and sampling was carried out on two occasions covering both wet and dry seasons, to account for potential seasonal variation in species abundance [75]; 20 traps × 24 days × 10 sites = 4800 trap-days in total).
Leaf litter ants were sampled at stations every 500 m along each transect. Leaf litter and loose topsoil were collected from five 1 m2 quadrats positioned 10 m apart (forming a cross shape) at each station (five quadrats × 5 stations × 11 sites = 275 quadrats in total). Quadrats were placed at least 10 m apart to ensure that sampled individuals were likely to have come from different nests [16,76,77]. Soil and leaf litter was sieved (mesh = 1 cm2) to remove debris and combined into a single sample per station. These samples were then emptied into mesh bags and hung inside modified Winkler bags in the shade for 3 days to extract ants [78], which were stored in 95 per cent alcohol.
(iii). Species identification
All trapped butterflies were identified where possible in the field (by S.B.; following Otsuka [79]), marked with a felt-tipped pen and released. Recaptured individuals were excluded from subsequent analyses. Individuals of the genera Tanaecia and Euthalia cannot be identified reliably in the field and were collected and identified in the laboratory using published keys and figures [79,80]; where necessary, this included dissection of male genitalia. All trapped moths were collected and identified in the laboratory (by S.B. and C.V.K.) using reference collections at the Forest Research Centre, Sabah, and published keys and figures [66–71].
Ants were identified (by N.T.) first to genus following existing keys [81,82], then to species using reference collections at the Natural History Museum (London), and Borneensis Collection (Universiti Malaysia Sabah), and online resources (http://www.antweb.org; http://www.antbase.net). Undescribed species that occur in www.antweb.org were given the same number as the online collection, and morphospecies not featured were given new reference numbers and submitted to the collection. Voucher specimens of ants and Lepidoptera have been deposited at the Forest Research Centre, Sandakan, Sabah.
(iv). Species richness and nestedness
Species richness is highly sensitive to sample size and even though sampling effort was standardized, sampling efficiency or total abundance may have varied across transects. The numbers of species recorded on different transects could therefore have reflected the numbers of individuals sampled rather than the numbers of species inhabiting different patches. Hence, we used a jackknife estimate to assess the probable species pool at each site (Smax ± 1 s.e.) from the number of species observed (Sobs), using the formula Smax = Sobs + a(n−1/n) where n is the number of samples and a the number of species in only one sample [83]. In addition to species richness at each study site, we also analysed the nestedness of assemblages across sites, using Binmatnest software (see above). Data from the two ‘control’ sites in continuous forest (Danum Valley and Maliau Basin) were combined prior to running Binmatnest. In keeping with previous studies (e.g. [27]), we restricted this analysis to species sampled five or more times in total, to increase the reliability of the presence/absence data in our species by site matrices. In addition, to check how robust our results were, we also used Nodf software [59] to analyse nestedness for all species sampled, using quantitative abundance-based matrices.
(v). Vegetation structure in fragments
To investigate relationships between nestedness and vegetation structure within each forest fragment, we measured the following variables at each sampling station (slightly different methods were used for ants and Lepidoptera). For Lepidoptera (n = 200 stations), we measured the height of the two nearest large trees (girth at breast height (g.b.h.) more than 60 cm) within 30 m in each of four quadrants centred at the station (maximum of eight trees per station), the number of quadrants with one or more trees within 30 m (maximum of four) and we estimated vegetation cover (%) at ground and canopy levels (see [27] for further details). For ants (n = 55 stations), we measured the girth (in cm) and distance (in m) of the eight nearest large trees (g.b.h. more than 60 cm) within 20 m of the centre of each station, and the number of these trees that were dipterocarps (which is a good indicator of the degree to which forest habitats within fragments are similar to undisturbed habitats within continuous forest [84]). We also measured leaf litter depth (cm) at each station (mean of five measurements, taken within each 1 m2 quadrat with a wooden rule prior to sampling of ants), and we estimated vegetation cover (%) at canopy and ground levels. Vegetation measurements were normalized where necessary (i.e. arcsine transformation of percentages) and analysed by principal components analysis (PCA [84]; Lepidoptera and ants were sampled at slightly different locations along transects and so the two datasets were analysed separately).
(vi). Ecological and life-history traits
To gauge the importance of stochastic effects in driving patterns of nestedness, we calculated the average abundance of each species of butterfly and moth, across those sites where each species occurred. We did not do this for ants, because individuals from each nest are highly aggregated and non-independent; hence, numbers of individuals sampled at a site are unlikely to indicate the probability of recording a species as present within other occupied sites. As an index of vagility, we measured butterfly forewing length in the field (±1 mm using vernier calipers), and obtained data on moth forewing length from published sources [66–71]. To assess the vulnerability of species to local extinction, butterflies and moths were assigned to four geographical range size groups ([27]; endemic to Borneo, restricted to Sundaland, restricted to the Oriental region, occurring beyond the Oriental region (using data from [66–71,85])). To examine host–plant diversity, we assigned species to two groups in terms of larval host plant specificity (narrow, all host plants within a single plant family; broad, exploits two or more families of host plants; data from [86]).
To examine how the nestedness of ant species was related to different ecological traits, sampled ants were assigned to one of four feeding guilds (predator, granivore, omnivore, scavenger) and four nesting site guilds (soil, leaf litter, decaying wood, generalist) using published information ([87] and the Australian Ant Online database (http://anic.ento.csiro.au/ants/). Ants were assigned to guilds at genus level because detailed information at species level is not currently available. We then analysed nestedness separately for each guild.
3. Results
(a). Avian responses to fragmentation
(i). Species–area relationships
Data were extracted from 26 published studies for subsequent re-analysis (see the electronic supplementary material, appendix S1). Studies examined between 3 and 35 fragments (median = 6 fragments), and all three major tropical regions were represented. Significant SARs were present in 13 out of 26 studies (50%; double-log power function, log S/log A), of which the range of slope values (z) was 0.048–0.81 and the range of intercept values (c) was 0.81–1.76 (untransformed: 6.5–57.5 ha). The number of fragments within a study was not significantly associated with any regression parameters (p > 0.05 in each case), and isolation did not explain a significant amount of variation in S beyond that accounted for by area (p > 0.05 in all cases).
However, studies with a wider range of isolation values among fragments had steeper slopes (rs = 0.36, p < 0.05) as did those at lower latitudes (rs = −0.57, p < 0.01). Studies from Africa had significantly shallower slopes than did studies from the Neotropics or Asia (Kruskal–Wallis: χ2 = 9.2, p < 0.01; Steel-type multiple comparison: p < 0.05). No other variables showed significant relationships with the slopes of SARs.
(ii). Nestedness
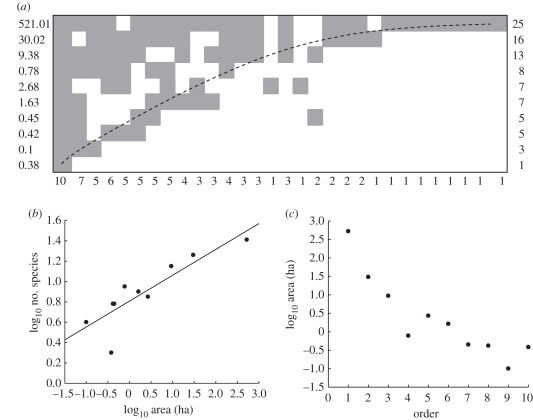
Data suitable for inclusion into nestedness analyses were obtained for 1570 records (1057 species) from 15 studies. Data on geographical range sizes were available for all species, and data on body masses were available for 876 species. All but two of the 15 studies were significantly nested, and nestedness index values (T) across studies were low (range = 5.0–35.3, mean ± 95% CI = 19.3 ± 6.3) indicating a high degree of nestedness in most cases (figure 2). For those studies showing nestedness, the rank order in which fragments were arranged to achieve maximum nestedness showed a significant negative relationship with fragment area in seven of 14 cases (figure 2c); a further five studies, mostly with few fragments, showed non-significant negative associations with area.
Figure 2.
The types of analyses we carried out on published datasets for birds. Data [88] showed significant patterns of species richness and composition among fragments. (a) Fragments arranged to achieve maximum nestedness of species presences (grey cells) and absences (white cells) in the fragment (rows) × species (columns) matrix. Values to the left of the matrix show fragment areas (ha), all other values show species richness. The matrix was significantly nested (T = 4.48, p < 0.001). (b) SAR fitted by the power function: log10S = log10 0.81 (s.e. ± 0.05) + 0.254 (s.e. ± 0.05) × log10 area. R2 = 0.76, p = 0.001. (c) The order in which fragments were arranged to achieve maximum nestedness was negatively correlated with their area (rs = −0.95, p < 0.001).
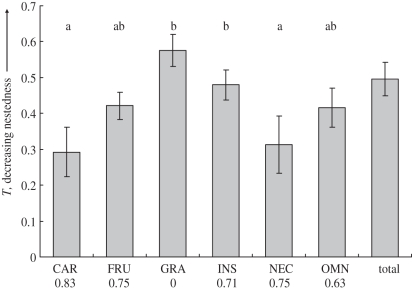
Studies that were more highly nested (i.e. lower T values) had steeper SAR slopes (rs = −0.65, p < 0.05) indicating that greater net rates of species loss following fragmentation were associated with more predictable sequences of species turnover. However, the degree of nestedness was not significantly related to the average isolation of fragments or to variation in sampling methodology or location of study (general linear model; p > 0.1 in all cases). Separate analysis for different subsets of species indicated that the degree of nestedness was significantly different among the six avian feeding guilds (χ2 = 11.1, p < 0.05) with carnivores and nectarivores significantly more nested than either granivores or insectivores (Steel-type multiple comparison: p < 0.01; figure 3). However, there was no significant difference in the nestedness of large and small bodied species (Mann–Whitney; z = 1.48, p = 0.2) or between species with large and small geographical ranges (z = 0.60, p = 0.6).
Figure 3.
The mean nestedness (T ± s.e.) of six feeding guilds and total assemblages of birds. Lower values of T indicate higher nestedness. Guilds are: CAR, carnivore; FRU, frugivore; GRA, granivore; INS, insectivore; NEC, nectarivore; OMN, omnivore. Guilds with the same letters are not significantly different at the 5% level. Numbers below guilds are the proportions of studies reporting significant (p < 0.05) nestedness.
(b). Responses of insects to forest fragmentation
(i). Differences in vegetation structure among sites
The PCA analyses of vegetation structure for sites where Lepidoptera and ants were sampled (n = 200 stations and 55 stations, respectively) produced one factor score for Lepidoptera sites (LEP-PRIN1) that accounted for 71 per cent of the variation in the data, and three independent factor scores for ant sites (ANT-PRIN1, ANT-PRIN2, ANT-PRIN3) that together explained 76 per cent of variation. For Lepidoptera sites, a high LEP-PRIN1 score represented tall dense forest with a closed canopy and little ground vegetation. For ant sites, a high ANT-PRIN1 score represented forest with many large trees, a high ANT-PRIN2 score represented forest with high canopy cover and many dipterocarps, and a high ANT-PRIN3 score represented forest with deep leaf litter and low ground cover. LEP-PRIN1 and ANT-PRIN2 were both positively related to fragment size (Spearman correlation, Lepidoptera, rs = 0.69, n = 10, p < 0.05; ants, rs = 0.61, n = 11, p < 0.05) showing that larger fragments were more likely to support closed-canopy forest with a high proportion of dipterocarp trees.
(ii). Species richness in forest fragments
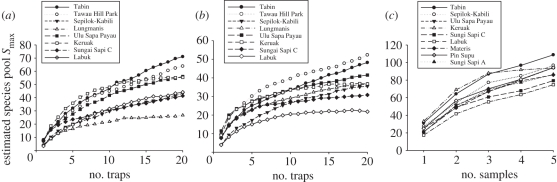
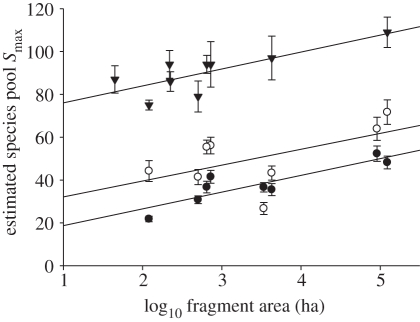
Excluding recaptures, we sampled 6833 individuals of 84 species of butterfly, 2112 individuals of 107 species of moth and 19 998 individuals of 200 species/morphospecies of ant (figure 4; see the electronic supplementary material, appendices S3 and S4). Estimation of Smax indicated that we sampled 77 per cent of the species present at each site for butterflies (range = 69–84%), 70 per cent for moths (range = 64–79%) and 71 per cent for ants (range = 67–81%). Excluding continuous forest locations, Smax for butterflies was significantly and positively related to log10 fragment area (figure 5) and negatively related to isolation (general linear model; F2,5 = 35.9, p = 0.001) but not to vegetation structure. For both moths and ants, there were significant positive relationships between Smax and log10 fragment area (figure 5; moths, F1,6 = 8.1 p < 0.05; ants, F1,6 = 13.4, p = 0.01) but no relationships with fragment isolation or vegetation structure (p > 0.3 in all cases). Comparison of SARs among the three insect taxa revealed very similar z values, although intercept values varied among taxa (species richness of butterflies < moths < ants; figure 5).
Figure 4.
Species accumulation curves showing changes in the estimated size of the species pool Smax with increasing sampling effort for (a) fruit-feeding moths (Noctuidae and Geometridae), (b) fruit-feeding butterflies (Nymphalidae) and (c) leaf-litter ants.
Figure 5.
Final estimated size of the species pool Smax ± 1 s.d. for leaf-litter ants (triangles) and fruit-feeding moths (open circles) and butterflies (solid circles) in rainforest fragments in Sabah, Borneo, in relation to log10 fragment area.
(iii). Patterns of nestedness
Excluding species with fewer than five individuals, we analysed patterns of nestedness for 48 of 84 recorded species of butterflies, 40 of 108 species of moths and 99 of 200 species of ants. The species occupancy data filled 49.4, 45.7 and 47.4 per cent of the overall species by site matrix for butterflies, moths and ants, respectively, and the observed temperatures of the maximally packed matrices (38.1°C, 31.7°C and 29.2°C, respectively) were significantly cooler than random (p < 0.01). Thus, all three insect taxa were significantly nested. The ant species data were then split according to feeding guild (four groups) and nesting locations (four groups), and the nestedness analysis was re-run separately for each group. Only predators were significantly nested (T = 15.0°C, n = 23 species, p = 0.01).
Examination of the species by site matrix showed that for butterflies, sites were nested by area, such that smaller sites contained species assemblages that were subsets of those present at larger sites (rs = −0.79, n = 9, p = 0.01), but this relationship was not evident in either moths or ants (p > 0.1). There was also no evidence for any insect taxon that sites were nested according to either site isolation or vegetation structure (p > 0.1). We also investigated whether the ordering of Lepidoptera in the species by site matrix was related to species traits. Moth species with large wings occurred at more sites, including those with few other species (rs = −0.58, n = 34, p < 0.001), but this effect was not evident in butterflies (rs = −0.25, n = 48, p = 0.1). In addition, there was some evidence that butterfly species with more restricted geographical ranges were confined mainly to those few sites with high species richness (Kruskal–Wallis χ2 = 5.30, n = 48, p = 0.07), but this effect was not evident in moths (χ2 = 3.5, n = 35, p = 0.3).
There was no relationship between nestedness and abundance in either moths (rs = −0.22, n = 40, p = 0.2) or butterflies (rs = −0.14, n = 48, p = 0.4). There were also no relationships with larval host plant specificity in either taxon (Mann–Whitney test of species matrix order by plant specificity; butterflies, z = 1.58, n = 48, p = 0.1; moths, z = 0.57, n = 26, p = 0.6). For both taxa, matrices generated using abundance data (using Nodf software) were very similar to those generated from presence–absence data (using Binmatnest software), resulting in very strong correlations in the ordering of both species and sites according to the two methods (ordering of species; butterflies, rs = 0.96, n = 48, p < 0.001; moths, rs = 0.95, n = 40, p < 0.001: ordering of sites; butterflies, rs = 0.95, n = 9, p < 0.001; moths, rs = 0.83, n = 9, p < 0.01). Consequently, there was no qualitative difference between methods for computing nestedness in the observed relationships with site characteristics or species traits.
4. Discussion
(a). Comparisons of SARs among taxa
Our analysis of 26 bird studies, and data for more than 390 insect species from 13 sites in Sabah revealed a general consensus in terms of species responses to habitat fragmentation. Significant SARs were common; approximately half of the bird studies and all three insect studies produced significant positive relationships between log10S and log10 area. The three insect taxa were studied in fragments from the same region and so it was perhaps not surprising that the SARs were very similar. Nonetheless, figure 5 shows that the slopes were almost identical across the three taxa, and the intercept values reflected differences in overall species richness between the groups (ants > moths > butterflies). Our studies on birds came from a heterogeneous dataset encompassing a wide range of study regions, matrix types and sizes and numbers of fragments. However, with the exception of region and latitude, variation in SARs in our bird studies was not related to these potentially confounding variables. Thus, we detected more variation in SARs within a single taxon sampled in different locations than we found among taxa studied at the same site. In our bird studies, we found no relationship between time-since-fragmentation and slope of SARs, implying that any differences in relaxation times of assemblages following fragmentation (i.e. extinction debt; [89]) did not affect the slopes of SARs. Previous studies have estimated that it takes approximately 50 years for half the species to be lost from a 1000 ha fragment [90]. Given the large sizes of many of our study fragments, it is likely that these fragments were not at equilibrium at the time of sampling, implying more extinctions from fragmented landscapes in future than were recorded by these studies.
In all taxa, area explained most of the variation in species richness (S) among fragments. However, in birds, the slope of the SARs (z) was steeper in studies that exhibited a great range of isolation values, and in butterflies, isolation explained additional variation in species richness after accounting for area. In addition, there was no effect of vegetation structure on SARs in any insect taxon. Thus, we conclude that extinction process were the most important drivers of species turnover following fragmentation, but that colonization processes were also evident.
(b). Nestedness patterns in different taxa
A high degree of nestedness was evident in all study taxa, and birds (mean = 19°C) were more nested than insects (mean = 33°C), implying more predictable patterns of species loss following fragmentation in birds. In insects there was no relationship between nestedness and abundance, indicating that observed patterns of nestedness were not an artefact of incomplete species inventories. In 50 per cent of the bird studies, and in butterflies, sites were nested by area. For all four study taxa, there was no suggestion that fragment isolation or vegetation structure of fragments affected the ranking of sites in the maximally ordered matrix. Thus, the findings from analyses of nestedness support those from analyses of SARs, and we conclude that extinction processes are most important in driving changes in species assemblages of birds and butterflies following fragmentation.
We found that in birds, more nested systems were associated with steeper SARs. This indicates that greater reduction in the number of species following fragmentation was associated with more predictable losses of species. Compared with insects, there was little evidence for new species of birds turning up in small fragments that were not present in larger areas. Compared with insects, birds may be more vulnerable to fragmentation effects, if they do not disperse across landscape matrices and so fail to colonize small fragments, or if fragments are too small to support viable populations of many species. Thus responses of birds to fragmentation may be less stochastic than those of insects.
Nestedness of birds and ants differed among feeding guilds, with carnivorous and nectivorous birds and predatory ants being the most nested whereas granivorous birds were the least nested, indicating that they may be less sensitive to reductions in forest area or more likely to colonize from the matrix [24]. These results are consistent with previous studies of birds on impacts of commercial selective logging [91,92] and fragmentation [53] showing that avian feeding guilds differed in their sensitivity to habitat disturbance, and that granivores were least affected. The sensitivity of avian carnivores and predatory ants to fragmentation may reflect their relatively high trophic status, for example, if their feeding requirements mean they have large minimum-area requirements for persistence. The sensitivity of avian nectarivores to habitat fragmentation that we detected may reflect that they are generally small specialist species with restricted ranges, which might be expected to be more adversely affected by fragmentation ([24,93]; but see [53]). In contrast, avian granivores and some guilds of ants may be able to exploit resources in matrix habitats [91].
(c). Relative importance of extinction processes
Our analyses of SARs and nestedness patterns in birds and insects following fragmentation allow us to determine the relative importance of different processes in driving changes in species richness and composition in fragments. Significant SARs with relatively little impact of fragment isolation, and no effect of vegetation structure on these relationships implies a primary role of extinction (i.e. area) on species richness. However, area was only an important driver in nestedness patterns in birds and butterflies, implying other factors were important for moths and ants.
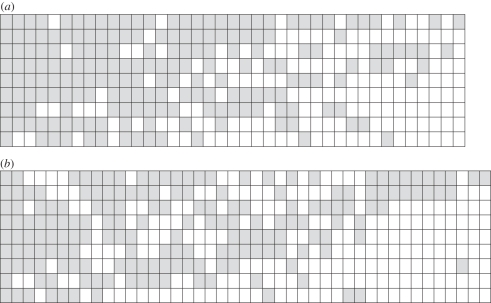
In insects, there was no evidence that the nestedness ranking of sites was associated with fragment isolation or vegetation structure, showing that colonization and habitat quality were not important drivers of nestedness. It could be that our data did not adequately capture the effects of forest fragmentation on habitat quality, although this seems unlikely given that there was a significant relationship between differences in vegetation structure and β-diversity of fruit-feeding butterflies among sites [27] (figure 6). The ranking of species of Lepidoptera in the maximally ordered site by species matrix showed that different traits were important for butterflies versus moths. For moths, species were nested by wing length showing that species with longer wings (which may be better dispersers [35,36]) occurred at a wide range of sites, including those small sites with few species present. This indicates that some moths are able to disperse across the agricultural matrix [94]. By contrast, butterfly species were not ranked by dispersal ability, but there was a trend for them to be nested by geographical range size, reflecting the fact that endemic species occurred only in the large, species-rich sites (area more than 4000 ha) and were not recorded in smaller fragments [27]. We interpret these results as evidence for colonization processes being important for moths, but extinction processes being more important for butterflies. Thus, even in two very closely related taxa from within a single feeding guild (fruit-feeding butterflies and moths), factors driving responses to fragmentation were different.
Figure 6.
Maximally ordered species presence–absence matrix for (a) fruit-feeding moths and (b) fruit-feeding butterflies in rainforest fragments in Sabah, Borneo. Filled squares indicate presence, white squares indicate absence; two species of moth and five species of butterfly present at all nine sites have been compressed into a single column.
(d). Implications for conservation and the value of small fragments
Our analyses showed that bird and insect assemblages in tropical forest fragments were highly nested, particularly in birds, such that species in small fragments were subsets of those in larger sites. However, responses of species were also idiosyncratic, particularly in insects where new species occurred in small fragments that had not been present in larger fragments. Thus, conservation of small fragments may be less important for birds than for insects in terms of conserving regional diversity. This supports findings from other studies for birds which concluded that small fragments were of little conservation value [95,96] whereas studies on insects and plants have highlighted the conservation value of small fragments [27,49,97,98].
While small fragments may support species not present in large fragments, differences among feeding guilds in patterns of nestedness following habitat fragmentation indicate that ecosystem functioning is likely to be altered in small fragments. For example, herbivory is reduced in small fragments [38–40,42], and the long-term viability of fragments may be reduced if seedling recruitment is affected [48].
Resources for conserving species are usually focused on large sites [99], but the idiosyncratic responses of some taxa to fragmentation indicate that the conservation of small fragments may promote regional diversity [27], and also increase productivity in surrounding agricultural areas [100,101]. Small fragments often experience increased habitat disturbance (this study; [27]) and altered micro-climates, and whether or not changes in trophic organization affect the long-term viability of these remnants requires further study.
Acknowledgements
We thank Glen Reynolds, the Royal Society South East Asia Rainforest Research Programme, Nazirah Mustaffa, and staff at Danum Valley Field Centre for logistical support. The project received funding from the UK Government Darwin Initiative (Defra) and the UK Biotechnology and Biological Sciences Research Council. This paper constitutes publication number A/578 of the Royal Society South East Asia Rainforest Research Programme.
References
- 1.Laurance W., Bierregaard R. (eds) 1997. Tropical forest remnants: ecology, management, and conservation of fragmented communities. Chicago, IL: University of Chicago [Google Scholar]
- 2.Groombridge B., Jenkins M. 2000. Global biodiversity: Earth's living resources in the 21st century. Cambridge, UK: World Conservation Press [Google Scholar]
- 3.Koh L. P., Wilcove D. S. 2008. Is oil palm agriculture really destroying tropical biodiversity? Conserv. Lett. 1, 60–64 10.1111/j.1755-263X.2008.00011.x (doi:10.1111/j.1755-263X.2008.00011.x) [DOI] [Google Scholar]
- 4.Peres C. A., Gardner T. A., Barlow J., Zuanon J., Michalski F., Lees A. C., Vieira I. C. G., Moreira F. M. S., Feeley K. J. 2010. Biodiversity conservation in human-modified Amazonian forest landscapes. Biol. Conserv. 143, 2314–2327 10.1016/j.biocon.2010.01.021 (doi:10.1016/j.biocon.2010.01.021) [DOI] [Google Scholar]
- 5.Brook B. W., Sodhi N. S., Ng P. K. L. 2003. Catastrophic extinctions follow deforestation in Singapore. Nature 424, 420–423 10.1038/nature01795 (doi:10.1038/nature01795) [DOI] [PubMed] [Google Scholar]
- 6.Fahrig L. 2003. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 34, 487–515 10.1146/annurev.ecolsys.34.011802.132419 (doi:10.1146/annurev.ecolsys.34.011802.132419) [DOI] [Google Scholar]
- 7.Laurance W. F., et al. 2002. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv. Biol. 16, 605–618 10.1046/j.1523-1739.2002.01025.x (doi:10.1046/j.1523-1739.2002.01025.x) [DOI] [Google Scholar]
- 8.Sodhi N. S., et al. 2010. Conserving South East Asian forest biodiversity in human-modified landscapes. Biol. Conserv. 143, 2375–2384 10.1016/j.biocon.2009.12.029 (doi:10.1016/j.biocon.2009.12.029) [DOI] [Google Scholar]
- 9.Bierregaard R., Gascon C., Lovejoy T., Mesquita R. (eds) 2001. Lessons from Amazonia: the ecology and conservation of a fragmented forest. Yale, UK: Yale University Press [Google Scholar]
- 10.Struebig M. J., Kingston T., Zubaid A., Mohd-Adnan A., Rossiter S. J. 2008. Conservation value of forest fragments to palaeotropical bats. Biol. Conserv. 141, 2112–2126 10.1016/j.biocon.2008.06.009 (doi:10.1016/j.biocon.2008.06.009) [DOI] [Google Scholar]
- 11.Klingbeil B. T., Willig M. R. 2010. Seasonal differences in population-, ensemble- and community-level responses of bats to landscape structure in Amazonia. Oikos 119, 1654–1664 10.1111/j.1600-0706.2010.18328.x (doi:10.1111/j.1600-0706.2010.18328.x) [DOI] [Google Scholar]
- 12.Charles J. K., Ang B. B. 2010. Non-volant small mammal community responses to fragmentation of Kerangas forests in Brunei Darussalam. Biodiv. Conserv. 19, 543–561 10.1007/s10531-009-9691-6 (doi:10.1007/s10531-009-9691-6) [DOI] [Google Scholar]
- 13.Bickford D., Ng T. H., Qie L., Kudavidanage E. P., Bradshaw C. J. A. 2010. Forest fragment and breeding habitat characteristics explain frog diversity and abundance in Singapore. Biotropica 42, 119–125 10.1111/j.1744-7429.2009.00542.x (doi:10.1111/j.1744-7429.2009.00542.x) [DOI] [Google Scholar]
- 14.Feeley K. J., Gillespie T. W., Lebbin D. J., Walter H. S. 2007. Species characteristics associated with extinction vulnerability and nestedness rankings of birds in tropical forest fragments. Anim. Conserv. 10, 493–501 10.1111/j.1469-1795.2007.00140.x (doi:10.1111/j.1469-1795.2007.00140.x) [DOI] [Google Scholar]
- 15.Shahabuddin G., Terborgh J. W. 1999. Frugivorous butterflies in Venezuelan forest fragments: abundance, diversity and the effects of isolation. J. Trop. Ecol. 15, 703–722 10.1017/S0266467499001121 (doi:10.1017/S0266467499001121) [DOI] [Google Scholar]
- 16.Bruhl C. A., Eltz T., Linsenmair K. E. 2003. Size does matter—effects of tropical rainforest fragmentation on the leaf litter ant community in Sabah, Malaysia. Biodiv. Conserv. 12, 1371–1389 10.1023/A:1023621609102 (doi:10.1023/A:1023621609102) [DOI] [Google Scholar]
- 17.Brosi B. J., Daily G. C., Shih T. M., Oviedo F., Duran G. 2008. The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 45, 773–783 10.1111/j.1365-2664.2007.01412.x (doi:10.1111/j.1365-2664.2007.01412.x) [DOI] [Google Scholar]
- 18.Davies R. G., Hernandez L. M., Eggleton P., Didham R. K., Fagan L. L., Winchester N. N. 2003. Environmental and spatial influences upon species composition of a termite assemblage across neotropical forest islands. J. Trop. Ecol. 19, 509–524 10.1017/s0266467403003560 (doi:10.1017/s0266467403003560) [DOI] [Google Scholar]
- 19.Kitching R. L., Orr A. G., Thalib L., Mitchell H., Hopkins M. S., Graham A. W. 2000. Moth assemblages as indicators of environmental quality in remnants of upland Australian rain forest. J. Appl. Ecol. 37, 284–297 10.1046/j.1365-2664.2000.00490.x (doi:10.1046/j.1365-2664.2000.00490.x) [DOI] [Google Scholar]
- 20.Didham R. K., Hammond P. M., Lawton J. H., Eggleton P., Stork N. E. 1998. Beetle species responses to tropical forest fragmentation. Ecol. Monogr. 68, 295–323 10.1890/0012-9615(1998)068[0295:BSRTTF]2.0.CO;2 (doi:10.1890/0012-9615(1998)068[0295:BSRTTF]2.0.CO;2) [DOI] [Google Scholar]
- 21.Rosenzweig M. 1995. Species diversity in space and time. Cambridge, UK: Cambridge University Press [Google Scholar]
- 22.Rodriguez-Girones M. A., Santamaria L. 2006. A new algorithm to calculate the nestedness temperature of presence–absence matrices. J. Biogeogr. 33, 924–935 10.1111/j.1365-2699.2006.01444.x (doi:10.1111/j.1365-2699.2006.01444.x) [DOI] [Google Scholar]
- 23.Louzada J., Gardner T., Peres C., Barlow J. 2010. A multi-taxa assessment of nestedness patterns across a multiple-use Amazonian forest landscape. Biol. Conserv. 143, 1102–1109 10.1016/j.biocon.2010.02.003 (doi:10.1016/j.biocon.2010.02.003) [DOI] [Google Scholar]
- 24.Fischer J., Lindenmayer D. B. 2005. Nestedness in fragmented landscapes: a case study on birds, arboreal marsupials and lizards. J. Biogeogr. 32, 1737–1750 10.1111/j.1365-2699.2005.01319.x (doi:10.1111/j.1365-2699.2005.01319.x) [DOI] [Google Scholar]
- 25.Lynam A. J., Billick I. 1999. Differential responses of small mammals to fragmentation in a Thailand tropical forest. Biol. Conserv. 91, 191–200 10.1016/S0006-3207(99)00082-8 (doi:10.1016/S0006-3207(99)00082-8) [DOI] [Google Scholar]
- 26.Laidlaw R. K. 2000. Effects of habitat disturbance and protected areas on mammals of peninsular Malaysia. Conserv. Biol. 14, 1639–1648 10.1046/j.1523-1739.2000.99073.x (doi:10.1046/j.1523-1739.2000.99073.x) [DOI] [PubMed] [Google Scholar]
- 27.Benedick S., Hill J. K., Mustaffa N., Chey V. K., Maryati M., Searle J. B., Schilthuizen M., Hamer K. C. 2006. Impacts of rain forest fragmentation on butterflies in Northern Borneo: species richness, turnover and the value of small fragments. J. Appl. Ecol. 43, 967–977 10.1111/j.1365-2664.2006.01209.x (doi:10.1111/j.1365-2664.2006.01209.x) [DOI] [Google Scholar]
- 28.Watling J. I., Gerow K., Donnelly M. A. 2009. Nested species subsets of amphibians and reptiles on neotropical forest islands. Anim. Conserv. 12, 467–476 10.1111/j.1469-1795.2009.00274.x (doi:10.1111/j.1469-1795.2009.00274.x) [DOI] [Google Scholar]
- 29.Wang Y. P., Bao Y. X., Yu M. J., Xu G. F., Ding P. 2010. Nestedness for different reasons: the distributions of birds, lizards and small mammals on islands of an inundated lake. Div. Distrib. 16, 862–873 10.1111/j.1472-4642.2010.00682.x (doi:10.1111/j.1472-4642.2010.00682.x) [DOI] [Google Scholar]
- 30.Wright D. H., Patterson B. D., Mikkelson G. M., Cutler A., Atmar W. 1998. A comparative analysis of nested subset patterns of species composition. Oecologia 113, 1–20 10.1007/s004420050348 (doi:10.1007/s004420050348) [DOI] [PubMed] [Google Scholar]
- 31.Moore R. P., Robinson W. D., Lovette I. J., Robinson T. R. 2008. Experimental evidence for extreme dispersal limitation in tropical forest birds. Ecol. Lett. 11, 960–968 10.1111/j.1461-0248.2008.01196.x (doi:10.1111/j.1461-0248.2008.01196.x) [DOI] [PubMed] [Google Scholar]
- 32.Frick W. F., Hayes J. P., Heady P. A. 2009. Nestedness of desert bat assemblages: species composition patterns in insular and terrestrial landscapes. Oecologia 158, 687–697 10.1007/s00442-008-1168-x (doi:10.1007/s00442-008-1168-x) [DOI] [PubMed] [Google Scholar]
- 33.Thomas C. D. 1991. Habitat use and geographic ranges of butterflies from the wet lowlands of Costa Rica. Biol. Conserv. 55, 269–281 10.1016/0006-3207(91)90032-5 (doi:10.1016/0006-3207(91)90032-5) [DOI] [Google Scholar]
- 34.Lozada T., de Koning G. H. J., Kessler M., Klein A. M., Tscharntke T. 2008. Geographical range size of tropical plants influences their response to anthropogenic activities. Div. Distrib. 14, 59–68 10.1111/j.1472-4642.2007.00413.x (doi:10.1111/j.1472-4642.2007.00413.x) [DOI] [Google Scholar]
- 35.Hughes C. L., Dytham C., Hill J. K. 2007. Modelling and analysing evolution of dispersal in populations at expanding range boundaries. Ecol. Entomol. 32, 437–445 10.1111/j.1365-2311.2007.00890.x (doi:10.1111/j.1365-2311.2007.00890.x) [DOI] [Google Scholar]
- 36.Berwaerts K., Van Dyck H., Aerts P. 2002. Does flight morphology relate to flight performance? An experimental test with the butterfly Pararge aegeria. Funct. Ecol. 16, 484–491 10.1046/j.1365-2435.2002.00650.x (doi:10.1046/j.1365-2435.2002.00650.x) [DOI] [Google Scholar]
- 37.Morris R. J. 2010. Anthropogenic impacts on tropical forest biodiversity: a network structure and ecosystem functioning perspective. Phil. Trans. R. Soc. B 365, 3709–3718 10.1098/rstb.2010.0273 (doi:10.1098/rstb.2010.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold A. E., Asquith N. M. 2002. Herbivory in a fragmented tropical forest: patterns from islands at Lago Gatun, Panama. Biodiv. Conserv. 11, 1663–1680 10.1023/A:1016888000369 (doi:10.1023/A:1016888000369) [DOI] [Google Scholar]
- 39.Ruiz-Guerra B., Guevara R., Mariano N. A., Dirzo R. 2010. Insect herbivory declines with forest fragmentation and covaries with plant regeneration mode: evidence from a Mexican tropical rain forest. Oikos 119, 317–325 10.1111/j.1600-0706.2009.17614.x (doi:10.1111/j.1600-0706.2009.17614.x) [DOI] [Google Scholar]
- 40.Savilaakso S., Koivisto J., Veteli T. O., Roininen H. 2009. Microclimate and tree community linked to differences in lepidopteran larval communities between forest fragments and continuous forest. Div. Distrib. 15, 356–365 10.1111/j.1472-4642.2008.00542.x (doi:10.1111/j.1472-4642.2008.00542.x) [DOI] [Google Scholar]
- 41.Faveri S. B., Vasconcelos H. L., Dirzo R. 2008. Effects of Amazonian forest fragmentation on the interaction between plants, insect herbivores, and their natural enemies. J. Trop. Ecol. 24, 57–64 10.1017/s0266467407004592 (doi:10.1017/s0266467407004592) [DOI] [Google Scholar]
- 42.Lopez L., Terborgh J. 2007. Seed predation and seedling herbivory as factors in tree recruitment failure on predator-free forested islands. J. Trop. Ecol. 23, 129–137 10.1017/s0266467406003828 (doi:10.1017/s0266467406003828) [DOI] [Google Scholar]
- 43.Herrerias-Diego Y., Quesada M., Stoner K. E., Lobo J. A., Hernandez-Flores Y., Montoya G. S. 2008. Effect of forest fragmentation on fruit and seed predation of the tropical dry forest tree Ceiba aesculifolia. Biol. Conserv. 141, 241–248 10.1016/j.biocon.2007.09.017 (doi:10.1016/j.biocon.2007.09.017) [DOI] [Google Scholar]
- 44.Dyer L. A., Letourneau D. K. 1999. Relative strengths of top-down and bottom-up forces in a tropical forest community. Oecologia 119, 265–274 10.1007/s004420050785 (doi:10.1007/s004420050785) [DOI] [PubMed] [Google Scholar]
- 45.Nichols E., Gardner T. A., Peres C. A., Spector S., Scarabaeinae Research Network 2009. Co-declining mammals and dung beetles: an impending ecological cascade. Oikos 118, 481–487 10.1111/j.1600-0706.2009.17268.x (doi:10.1111/j.1600-0706.2009.17268.x) [DOI] [Google Scholar]
- 46.Terborgh J., et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 10.1126/science.1064397 (doi:10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 47.Benitez-Malvido J. 1998. Impact of forest fragmentation on seedling abundance in a tropical rain forest. Conserv. Biol. 12, 380–389 10.1046/j.1523-1739.1998.96295.x (doi:10.1046/j.1523-1739.1998.96295.x) [DOI] [Google Scholar]
- 48.Cordeiro N. J., Howe H. F. 2003. Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. Proc. Natl Acad. Sci. USA 100, 14 052–14 056 10.1073/pnas.2331023100 (doi:10.1073/pnas.2331023100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner I. M., Corlett R. T. 1996. The conservation value of small, isolated fragments of lowland tropical rain forest. Trends Ecol. Evol. 11, 330–333 10.1016/0169-5347(96)10046-X (doi:10.1016/0169-5347(96)10046-X) [DOI] [PubMed] [Google Scholar]
- 50.Klein A. M. 2009. Nearby rainforest promotes coffee pollination by increasing spatio-temporal stability in bee species richness. Forest Ecol. Manage. 258, 1838–1845 10.1016/j.foreco.2009.05.005 (doi:10.1016/j.foreco.2009.05.005) [DOI] [Google Scholar]
- 51.Ricketts T. H. 2004. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv. Biol. 18, 1262–1271 10.1111/j.1523-1739.2004.00227.x (doi:10.1111/j.1523-1739.2004.00227.x) [DOI] [Google Scholar]
- 52.Sigel B. J., Sherry T. W., Young B. E. 2006. Avian community response to lowland tropical rainforest isolation: 40 years of change at La Selva biological station, Costa Rica. Conserv. Biol. 20, 111–121 10.1111/j.1523-1739.2006.00293 (doi:10.1111/j.1523-1739.2006.00293) [DOI] [PubMed] [Google Scholar]
- 53.Kennedy C. M., Marra P. P., Fagan W. F., Neel M. C. 2010. Landscape matrix and species traits mediate responses of neotropical resident birds to forest fragmentation in Jamaica. Ecol. Monogr. 80, 651–669 10.1890/09-0904.1 (doi:10.1890/09-0904.1) [DOI] [Google Scholar]
- 54.Perfecto I., Mas A., Dietsch T., Vandermeer J. 2003. Conservation of biodiversity in coffee agroecosystems: a tri-taxa comparison in southern Mexico. Biodiv. Conserv. 12, 1239–1252 10.1023/A:1023039921916 (doi:10.1023/A:1023039921916) [DOI] [Google Scholar]
- 55.Lewis O. T. 2009. Biodiversity change and ecosystem function in tropical forests. Basic Appl. Ecol. 10, 97–102 10.1016/j.baae.2008.08.010 (doi:10.1016/j.baae.2008.08.010) [DOI] [Google Scholar]
- 56.Gotelli N., Entsminger G. 2006. ECOSIM: null models software for ecology, Version 7.0. Jericho, VT: Acquired Intelligence Inc. & Kesey-Bear [Google Scholar]
- 57.Crawley M. 2007. The R book. New York, NY: Wiley [Google Scholar]
- 58.Atmar W., Patterson B. D. 1993. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96, 373–382 10.1007/BF00317508 (doi:10.1007/BF00317508) [DOI] [PubMed] [Google Scholar]
- 59.Almeida-Neto M., Ulrich W. 2011. A straightforward computational approach for measuring nestedness using quantitative matrices. Environ. Modell. Softw. 26, 173–178 10.1016/j.envsoft.2010.08.003 (doi:10.1016/j.envsoft.2010.08.003) [DOI] [Google Scholar]
- 60.Dunning J. 1992. CRC handbook of avian body masses. Belhaven, NC: CRC Press [Google Scholar]
- 61.Benedick S., White T. A., Searle J. B., Hamer K. C., Mustaffa N., Vun Khen C., Mohamed M., Schilthuizen M., Hill J. K. 2007. Impacts of habitat fragmentation on genetic diversity in a tropical forest butterfly on Borneo. J. Trop. Ecol. 23, 623–634 10.1017/s0266467407004543 (doi:10.1017/s0266467407004543) [DOI] [Google Scholar]
- 62.Koh L. P., Sodhi N. S., Tan H. T. W., Peh K. S. H. 2002. Factors affecting the distribution of vascular plants, springtails, butterflies and birds on small tropical islands. J. Biogeogr. 29, 93–108 10.1046/j.1365-2699.2002.00657.x (doi:10.1046/j.1365-2699.2002.00657.x) [DOI] [Google Scholar]
- 63.Didham R. K. 1997. The influence of edge effects and forest fragmentation on leaf litter invertebrates in central Amazonia. In Tropical forest remnants: ecology, management and conservation of fragmented communities (eds Laurance W. F., Bierregaard R. O., Jr), pp. 55–70 Chicago, IL: University of Chicago Press [Google Scholar]
- 64.Hill J. K., Hamer K. C., Tangah J., Dawood M. 2001. Ecology of tropical butterflies in rainforest gaps. Oecologia 128, 294–302 10.1007/s004420100651 (doi:10.1007/s004420100651) [DOI] [PubMed] [Google Scholar]
- 65.Hamer K. C., Hill J. K., Benedick S., Mustaffa N., Chey V. K., Maryati M. 2006. Diversity and ecology of carrion- and fruit-feeding butterflies in Bornean rain forest. J. Trop. Ecol. 22, 25–33 10.1017/s0266467405002750 (doi:10.1017/s0266467405002750) [DOI] [Google Scholar]
- 66.Holloway J. 1983. The moths of Borneo. Part 14: Noctuidae. Malayan Nat. J. 38, 157–317 [Google Scholar]
- 67.Holloway J. 1989. The moths of Borneo. Part 12: Noctuidae. Kuala Lumpur, Malaysia: The Malaysian Nature Society [Google Scholar]
- 68.Holloway J. 1993. The moths of Borneo. Part 11: Geometridae. Kuala Lumpur, Malaysia: The Malaysian Nature Society [Google Scholar]
- 69.Holloway J. 1996. The moths of Borneo. Part 9: Geometridae. Malayan Nat. J. 49, 147–326 [Google Scholar]
- 70.Holloway J. 1997. The moths of Borneo. Part 10: Geometridae. Malayan Nat. J. 51, 1–242 [Google Scholar]
- 71.Holloway J. 2005. The moths of Borneo. Parts 15 & 16 : Noctuidae, subfamily Catocalinae. Malayan Nat. J. 58, 1–529 [Google Scholar]
- 72.Chen I. C., Shiu H. J., Benedick S., Holloway J. D., Cheye V. K., Barlow H. S., Hill J. K., Thomas C. D. 2009. Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc. Natl Acad. Sci. USA 106, 1479–1483 10.1073/pnas.0809320106 (doi:10.1073/pnas.0809320106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen I. C., Hill J. K., Shiu H. J., Holloway J. D., Benedick S., Chey V. K., Barlow H. S., Thomas C. D. 2011. Asymmetric boundary shifts of tropical montane Lepidoptera over four decades of climate warming. Global Ecol. Biogeogr. 20, 34–45 10.1111/j.1466-8238.2010.00594.x (doi:10.1111/j.1466-8238.2010.00594.x) [DOI] [Google Scholar]
- 74.Dumbrell A. J., Hill J. K. 2005. Impacts of selective logging on canopy and ground assemblages of tropical forest butterflies: implications for sampling. Biol. Conserv. 125, 123–131 10.1016/j.biocon.2005.02.016 (doi:10.1016/j.biocon.2005.02.016) [DOI] [Google Scholar]
- 75.Hamer K. C., Hill J. K., Mustaffa N., Benedick S., Sherratt T. N., Chey V. K., Maryati M. 2005. Temporal variation in abundance and diversity of butterflies in Bornean rain forests: opposite impacts of logging recorded in different seasons. J. Trop. Ecol. 21, 417–425 10.1017/S0266467405002361 (doi:10.1017/S0266467405002361) [DOI] [Google Scholar]
- 76.Kaspari M. 1993. Body-size and microclimate use in neotropical granivorous ants. Oecologia 96, 500–507 10.1007/BF00320507 (doi:10.1007/BF00320507) [DOI] [PubMed] [Google Scholar]
- 77.Woodcock P., Edwards D. P., Fayle T. M., Newton R. J., Vun Khen C., Bottrell S. H., Hamer K. C. 2011. The conservation value of South East Asia's highly degraded forests: evidence from leaf-litter ants. Phil. Trans. R. Soc. B 366, 3256–3264 10.1098/rstb.2011.0031 (doi:10.1098/rstb.2011.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bestelmeyer B. T. 2000. The trade-off between thermal tolerance and behavioural dominance in a subtropical South American ant community. J. Anim. Ecol. 69, 998–1009 10.1046/j.1365-2656.2000.00455.x (doi:10.1046/j.1365-2656.2000.00455.x) [DOI] [Google Scholar]
- 79.Otsuka K. 1988. The butterflies of Borneo. Tokyo, Japan: Tobishima Corporation [Google Scholar]
- 80.Corbet A., Pendlebury H. 1992. The butterflies of the Malay peninsula, 4th edn. Kuala Lumpur, Malaysia: Malayan Nature Society [Google Scholar]
- 81.Hashimoto Y. 2003. Identification guide to ant genera of Borneo. See http://www.Antweb.Org/borneo.Jsp
- 82.Bolton B. 1994. Identification guide to ant genera of the world. Harvard, UK: University Press [Google Scholar]
- 83.Heltshe J. F., Forrester N. E. 1983. Estimating diversity using quadrat sampling. Biometrics 39, 1073–1076 10.2307/2531340 (doi:10.2307/2531340) [DOI] [Google Scholar]
- 84.Hamer K. C., Hill J. K., Benedick S., Mustaffa N., Sherratt T. N., Maryati M. K., Chey V. 2003. Ecology of butterflies in natural and selectively logged forests of Northern Borneo: the importance of habitat heterogeneity. J. Appl. Ecol. 40, 150–162 10.1046/j.1365-2664.2003.00783.x (doi:10.1046/j.1365-2664.2003.00783.x) [DOI] [Google Scholar]
- 85.Tsukada E. 1982. Butterflies of the south east Asian islands. Tokyo, Japan: Plapac Company [Google Scholar]
- 86.Robinson G., Ackery P., Kitching I., Beccaloni G., Hernandez L. 2001. Hostplants of the moths and butterfly caterpillars of the oriental region. London, UK: The Natural History Museum [Google Scholar]
- 87.Agositi D., Mayer J., Alonso L., Schultz E. 2000. Ants: standard methods for measuring and monitoring biodiversity. Washington, DC: Smithsonian Institution Press [Google Scholar]
- 88.Newmark W. D. 1991. Tropical forest fragmentation and the local extinction of understory birds in the eastern Usambara mountains, Tanzania. Conserv. Biol. 5, 67–78 10.1111/j.1523-1739.1991.tb00389.x (doi:10.1111/j.1523-1739.1991.tb00389.x) [DOI] [Google Scholar]
- 89.Tilman D., May R. M., Lehman C. L., Nowak M. A. 1994. Habitat destruction and the extinction debt. Nature 371, 65–66 10.1038/371065a0 (doi:10.1038/371065a0) [DOI] [Google Scholar]
- 90.Brooks T. M., Pimm S. L., Oyugi J. O. 1999. Time lag between deforestation and bird extinction in tropical forest fragments. Conserv. Biol. 13, 1140–1150 10.1046/j.1523-1739.1999.98341.x (doi:10.1046/j.1523-1739.1999.98341.x) [DOI] [Google Scholar]
- 91.Gray M. A., Baldauf S. L., Mayhew P. J., Hill J. K. 2007. The response of avian feeding guilds to tropical forest disturbance. Conserv. Biol. 21, 133–141 10.1111/j.1523-1739.2006.00557.x (doi:10.1111/j.1523-1739.2006.00557.x) [DOI] [PubMed] [Google Scholar]
- 92.Edwards D. P., Ansell F. A., Ahmad A. H., Nilus R., Hamer K. C. 2009. The value of rehabilitating logged rainforest for birds. Conserv. Biol. 23, 1628–1633 10.1111/j.1523-1739.2009.01330.x (doi:10.1111/j.1523-1739.2009.01330.x) [DOI] [PubMed] [Google Scholar]
- 93.Mac Nally R., Bennett A. F. 1997. Species-specific predictions of the impact of habitat fragmentation: local extinction of birds in the box-ironbark forests of central Victoria, Australia. Biol. Conserv. 82, 147–155 10.1016/S0006-3207(97)00028-1 (doi:10.1016/S0006-3207(97)00028-1) [DOI] [Google Scholar]
- 94.Ricketts T. H., Daily G. C., Ehrlich P. R., Fay J. P. 2001. Countryside biogeography of moths in a fragmented landscape: biodiversity in native and agricultural habitats. Conserv. Biol. 15, 378–388 10.1046/j.1523-1739.2001.015002378.x (doi:10.1046/j.1523-1739.2001.015002378.x) [DOI] [Google Scholar]
- 95.Edwards D. P., Hodgson J. A., Hamer K. C., Mitchell S. L., Ahmad A. H., Cornell S. J., Wilcove D. S. 2010. Wildlife-friendly oil palm plantations fail to protect biodiversity effectively. Conserv. Lett. 3, 236–242 10.1111/j.1755-263X.2010.00107.x (doi:10.1111/j.1755-263X.2010.00107.x) [DOI] [Google Scholar]
- 96.Beier P., Van Drielen M., Kankam B. O. 2002. Avifaunal collapse in west African forest fragments. Conserv. Biol. 16, 1097–1111 10.1046/j.1523-1739.2002.01003.x (doi:10.1046/j.1523-1739.2002.01003.x) [DOI] [Google Scholar]
- 97.Klein A. M., Steffan-Dewenter I., Tscharntke T. 2006. Rain forest promotes trophic interactions and diversity of trap-nesting Hymenoptera in adjacent agroforestry. J. Anim. Ecol. 75, 315–323 10.1111/j.1365-2656.2006.01042.x (doi:10.1111/j.1365-2656.2006.01042.x) [DOI] [PubMed] [Google Scholar]
- 98.Veddeler D., Schulze C. H., Steffan-Dewenter I., Buchori D., Tscharntke T. 2005. The contribution of tropical secondary forest fragments to the conservation of fruit-feeding butterflies: effects of isolation and age. Biodiv. Conserv. 14, 3577–3592 10.1007/s10531-004-0829-2 (doi:10.1007/s10531-004-0829-2) [DOI] [Google Scholar]
- 99.Primack R. 2006. Essentials of conservation biology, 4th edn. Boston, MA: Sinauer Associates Ltd [Google Scholar]
- 100.Rand T. A., Tylianakis J. M., Tscharntke T. 2006. Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 9, 603–614 10.1111/j.1461-0248.2006.00911.x (doi:10.1111/j.1461-0248.2006.00911.x) [DOI] [PubMed] [Google Scholar]
- 101.Ricketts T. H., Daily G. C., Ehrlich P. R., Michener C. D. 2004. Economic value of tropical forest to coffee production. Proc. Natl Acad. Sci. USA 101, 12 579–12 582 10.1073/pnas.0405147101 (doi:10.1073/pnas.0405147101) [DOI] [PMC free article] [PubMed] [Google Scholar]