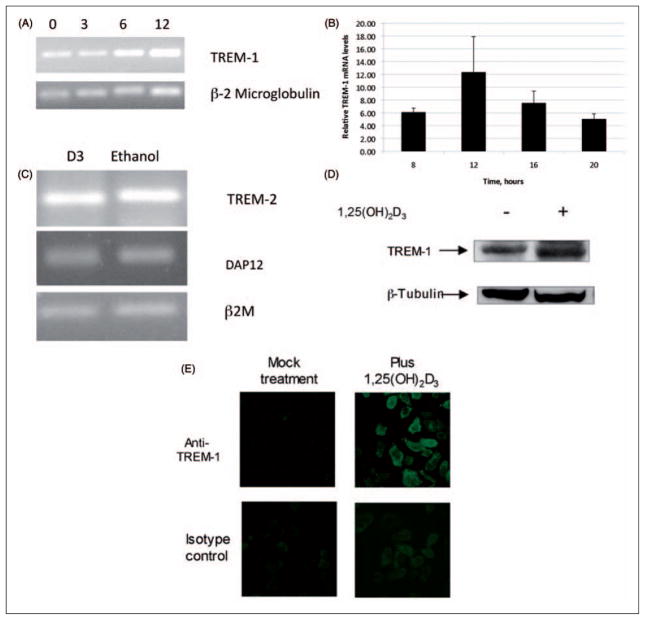
Figure 1.
Induction of TREM-1 in airway epithelial cells in response to 1,25(OH)2D3. The normal human bronchial epithelial (NHBE) cells were grown in air-liquid interface (ALI) and stimulated with 10−8 M 1,25(OH)2D3 or ethanol in the basolateral medium for increasing times from 0–12 h (A,B). Total mRNA was isolated and TREM-1 mRNA levels were observed by RT-PCR followed by gel electrophoresis (A). The experiment was repeated and the mRNA was quantified by QPCR (B). Results in B are shown as levels relative to ethanol-treated samples at each timepoint. The mRNA levels are relative to β-2 microglobulin (n = 3; bars indicate mean ± SD). The increase in TREM-1 mRNA in basolaterally stimulated cultures is significant at all timepoints, as determined by t-test (P <0.001). (C) RT-PCR analysis of TREM-2 and DAP 12 in cultures stimulated with either 10−8 M 1,25(OH)2D3 or 0.01% ethanol for 18 h. (D). The TREM-1 protein levels were visualized by western blot analysis of whole cell lysates of NHBE cells cultured in ALI, and stimulated with 1,25(OH)2D3 for 18 h. (E) Surface expression of TREM-1 was observed by stimulation of NHBE cells grown on chamber slides in the presence of 10−8 M 1,25(OH)2D3 or 0.01% ethanol for 24 h. Slides were fixed and incubated with anti-TREM-1 antibody, followed by an Alexa-Fluor conjugated secondary antibody, and visualized by fluorescence microscopy.