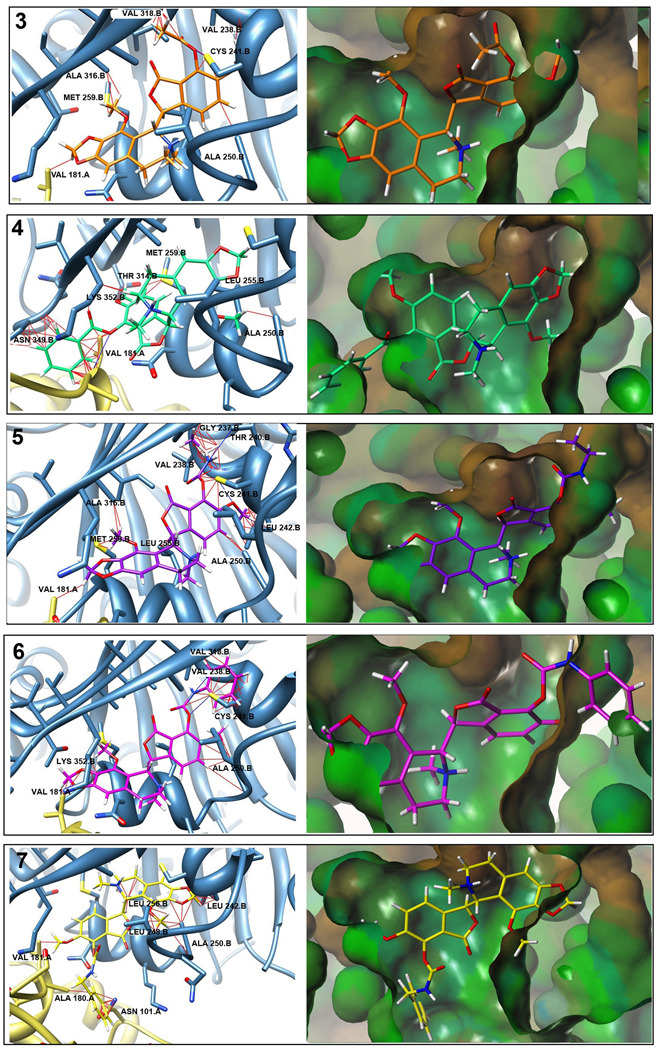
Fig. 4.
Predicted noscapinoid binding positions on tubulin. Panels on left show predicted binding position of noscapinoids based upon their superpositioning on DAMA-colchicine from colchicine-tubulin crystal structure and then overlay into the protein [27]. Potential steric clashes (red lines) and H-bonds (blue lines) as predicted by UCSF Chimera are shown. Larger substitutions at the 7-position show increased steric hindrances versus those of noscapine. Panels on right show noscapinoids in the hydrophobic surface of the colchicine binding pocket created using Tripos Sybyl. Steric clashes can be seen in portions of the molecules shown outside of the pocket.