Abstract
Osteoclasts are large, multinucleated cells responsible for the resorption of mineralized bone matrix. These cells are critical players in the bone turnover involved in bone homeostasis. Osteoclast activity is connected to the establishment and expansion of skeletal metastases from a number of primary neoplasms. Thus, the formation and activation of osteoclasts is an area of research with many potential avenues for clinical translation. Past studies of osteoclast biology have utilized primary murine cells cultured in vitro. Recently, techniques have been described that involve the generation of osteoclasts from human precursor cells. However, these protocols are often time-consuming and insufficient for generating large numbers of osteoclasts. We therefore developed a simplified protocol by which human osteoclasts may be easily and reliably generated in large numbers in vitro. In this study, osteoclasts were differentiated from bone marrow cells that had been aliquotted and frozen. Cells were generated by culture with recombinant macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL). Both human and murine RANKL were shown to efficiently generate osteoclasts, although higher concentrations of murine RANKL were required. Formation of osteoclasts was demonstrated qualitatively by tartrate-resistant acid phosphatase (TRAP) staining. These cells were fully functional, as confirmed by their ability to form resorption pits on cortical bone slices. Functional human osteoclasts can be difficult to generate in vitro by current protocols. We have demonstrated a simplified system for the generation of human osteoclasts in vitro that allows for large numbers of osteoclasts to be obtained from a single donor.
Keywords: Osteoclast; protocol; bone resorption; human, bone marrow; in vitro
Introduction
Osteoclasts are giant, multinucleated cells of hemopoietic lineage that are unique in their ability to resorb bone. Osteoclast differentiation requires the stimulation of precursors by macrophage colony-stimulating factor (M-CSF) as well as the binding of receptor activator of NF-kB ligand (RANKL) to its receptor RANK on the cell surface [1–3]. Osteoclast biology is an active area of investigation in which research promises to have important implications for many aspects of human disease, ranging from osteoporosis to metastatic cancer. Many studies of osteoclast biology have relied on the generation of osteoclasts from primary murine cells, protocols for which have been described [4]. Although studies employing murine cells have yielded much valuable information, we suggest that studies based on human cells will more readily translate into clinical benefits. However, the generation of osteoclasts from human cells can be more challenging than that from murine cells. In general, protocols for the generation of human osteoclasts in vitro rely on the isolation of peripheral blood mononuclear cells (PBMCs) to serve as precursors. The protocols described by Nardelli et al. and Susa et al. are perhaps typical of this approach [5, 6]. Other investigators have described the generation of osteoclasts from peritoneal macrophages [7], cord blood [8], and bone marrow [8–12]. Even among protocols that employ bone marrow cells, variations exist with respect to the specific cell populations employed, the supplements required, and the timeframe needed. Typically, these protocols involve isolating mononuclear cells from fresh bone marrow by density gradient centrifugation and the subsequent plating of these cells in tissue culture flasks or plates. The entirety of these cells is then cultured under osteoclastogenic conditions [7–15]. In some cases, after an initial incubation period in tissue culture flasks, nonadherent cells are discarded and only the adherent cells are kept in culture [16, 17]. Because the adherent cell population includes bone marrow stromal cells, these cultures are in fact co-cultures, and can be supplemented with 1,25-dihydroxyvitamin D3 alone [9, 10, 16], or in combination with M-CSF, which increases the efficiency of the yield [12, 18], and dexamethasone [7, 13]. In other reports, nonadherent cells are harvested to serve as osteoclast precursors, following a 24-hour incubation in tissue culture flasks [8, 19]. These cultures are supplemented with RANKL alone [19] or M-CSF and RANKL [8]. In some studies, immunomagnetic cell separation is used to isolate specific cell populations, such as CD14+ or CD34+ [6, 15] or to remove unwanted populations (CD3+, CD19+) [8]. Co-cultures of human bone marrow mononuclear cells and rodent stromal cell lines have also been described [7, 14, 15]. It is clear, in consideration of the above, that human osteoclasts may be generated in vitro by a diverse array of strategies.
The variety of protocols detailed above may confound attempts to generalize observations made in a particular study. Thus, a standardized protocol for osteoclast generation would be desirable. An ideal protocol would involve relatively simple procedures, require few supplements, and necessitate a comparatively short amount of time to complete. Furthermore, it would rely on easily obtainable source material that could be used to establish a stock from which many future experiments could be derived. Here, we describe a simplified system for the in vitro generation of human osteoclasts that satisfies the above requirements.
Materials and methods
Preparation of cells
Human bone marrow macrophages were prepared from fresh human bone marrow, purchased from Lonza (Lonza Walkersville, Walk-ersville, MD). Upon arrival, the marrow was diluted with an equal volume of 20% (v/v) dimethyl sulfoxide (Fisher Scientific, Pittsburgh, PA) in FBS and frozen in 2 ml aliquots at -80 °C overnight, before being stored in liquid nitrogen. Prior to use, aliquots were thawed at 37 °C and washed twice in 20 ml α-MEM followed by centrifugation at 400 x g for 5 min. Cells were then resuspended in 10 ml α-MEM and layered over 8 ml lymphocyte separation medium (density 1.077 g/ml; Mediatech, Manassas, VA) in a 50 ml conical tube before being centrifuged for 25 min at 16 °C. Following centrifugation, the white cell layer at the interface was harvested and washed twice as above. The cells were resuspended in a final volume of 10 ml α-MEM containing 10% FBS (v/v), supplemented with 35 ng/ml recombinant human M-CSF (R & D Systems, Inc., Minneapolis, MN) and cultured overnight in a 75-cm2 tissue culture flask. The following day, nonadherent cells were harvested and transferred to a 100 mm suspension plate. The medium was replaced with fresh medium containing 35 ng/ml human M-CSF every three days until the cells were used for experiments.
Osteoclastogenesis
Bone marrow macrophages were prepared from cryopreserved marrow and cultured for five days in a suspension plate, at which point both suspended and adherent cell populations existed. Suspended cells were harvested with the culture medium, while adherent cells were detached with 0.02% EDTA in PBS and incubated for 10 min at 37 °C. A 24-well tissue culture plate was seeded with cells from either adherent or nonadherent populations, or a 1:1 mixture of both, at a total of 1 x 105 cells per well. The cells were then cultured in medium containing 10 ng/ml M-CSF and 10, 50 or 100 ng/ml human RANKL. After plating cells, 1ml of medium (with supplements) was added on day two, as the cells had not yet become firmly attached to the plate. The medium was completely removed and replaced with fresh osteoclastogenic medium every 3 days thereafter. On day 10, the cells were stained for tartrate-resistant acid phosphatase (TRAP) with a leukocyte acid phosphatase kit (387-A; Sigma-Aldrich, St. Louis, MO).
Osteoclasts were also generated in medium supplemented with recombinant murine RANKL, which was prepared as previously described [20]. Cryopreserved cells were prepared for culture as above and grown in suspension plates for 14 days, at which point the adherent population was predominant. Adherent cells were detached with 0.02% EDTA as before, and a 48-well tissue culture plate was seeded with 1.7 x 104 cells per well. Cells were cultured with 12 ng/ml M-CSF and 100 ng/ml recombinant murine RANKL. Cells were stained for TRAP at day 11.
Resorption and microscopy
Cryopreserved cells were thawed and prepared for culture as above. After one day in suspension culture, 2 x 105 cells were placed into each well of a 24-well plate containing sterile, devitalized slices of bovine cortical bone measuring approximately 1 cm2. Cultures were maintained with 10 ng/ml M-CSF and 100 ng/ml murine RANKL Control wells received M-CSF but no RANKL. Cells were then cultured as above (fresh medium every 3 days). Bone slices were removed from culture on day 18, to be assayed for the formation of resorption pits. The slices were rinsed with water and then incubated for 10 min at room temperature with 0.25 M ammonium hydroxide. Slices were then rinsed with water, and cells were removed by mechanical agitation. Bone slices were then subjected to scanning electron microscopy (SEM) using a Philips 515 SEM (Materials Engineering Department, University of Alabama at Birmingham).
Results
Osteoclast formation
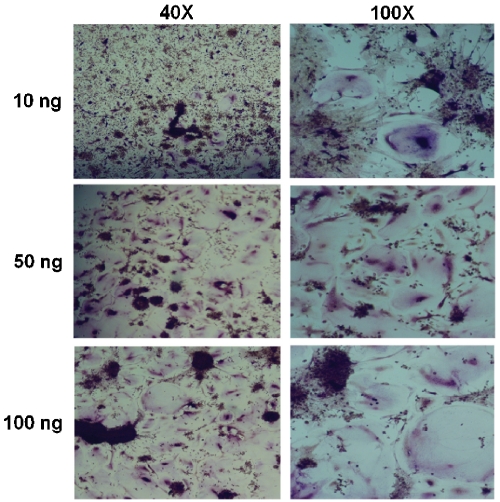
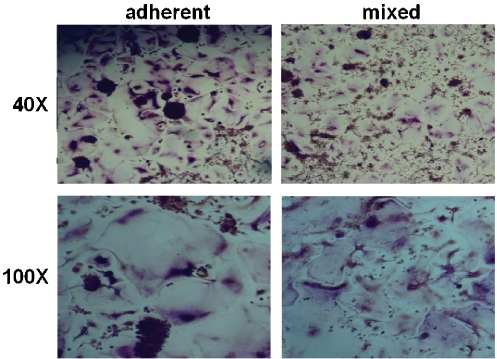
We intended to establish a stock for future experiments and thus wished to verify that human osteoclasts could be generated from cryopreserved bone marrow in this expedited protocol. Freshly harvested bone marrow was obtained from a commercial source, immediately aliquotted, and stored in liquid nitrogen. Samples were subsequently thawed, and an overnight incubation in tissue culture flasks was used to remove stromal cells from the population, as only cells that remained nonadherent after this period were utilized for osteoclastogenesis. These cells were then cultured in suspension dishes and maintained in the presence of 35 ng/ml human M-CSF to enrich the culture for pre-osteoclasts, until they were employed in osteoclastogenesis experiments. Under these conditions, we observed a gradual shift in phenotype as the cells, initially small, rounded, and nonadherent, began to weakly attach to the plate after several days, eventually becoming enlarged, flattened, and firmly adherent. We assayed the cells for osteoclastogenic potential after allowing preosteoclast enrichment for five days in culture, at which point both adherent and suspension populations of cells were present. Both populations of cells were seeded in 24-well plates and maintained with 10 ng/ml M-CSF and 10, 50 or 100 ng/ml human RANKL. Under these conditions, we observed osteoclastogenesis within 7-8 days. In contrast, cells cultured without RANKL did not form osteoclasts. The plates were stained for TRAP on day 10 to confirm the presence of mature osteoclasts in wells seeded with nonadherent cells (Figure 1). We observed more efficient osteoclast formation in medium supplemented with 50 ng/ml RANKL versus medium supplemented with 10 ng/ml, while increasing the concentration to 100 ng/ml did not further increase the osteoclast yield. We observed similar osteoclas-togenic potential from both the adherent precursor cells and a 1:1 mixture of adherent and nonadherent cells (Figure 2), therefore all subsequent experiments relied on both populations in aggregate.
Figure 1.
Human osteoclasts can be generated from nonadherent, cryopreserved bone marrow macrophages in a simplified protocol. Human bone marrow macrophages were isolated from whole bone marrow following cryopreservation. Cells that remained nonadherent after 5 days in suspension culture were cultivated in the presence of 10 ng/ml recombinant human M-CSF and varying concentrations of recombinant human RANKL, as indicated. Large, multinu-cleated cells became visible after 8 days, and cultures were stained for the presence of TRAP after 10 days. Shown are representative micrographs at 40X (left column) and 100X (right column).
Figure 2.
Human osteoclasts can be generated from both adherent and mixed adherent/nonadherent precursors. Adherent (left column) or a 1:1 mixture (right column) of adherent and nonadherent bone marrow macrophages were harvested after 5 days in suspension culture and seeded in a tissue culture plate. Cells were grown in the presence of 10 ng/ml recombinant human M-CSF and 50 ng/ml recombinant human RANKL for 10 days. Shown are cells stained for the presence of TRAP at magnifications of 40X (top row) and 100X (bottom row).
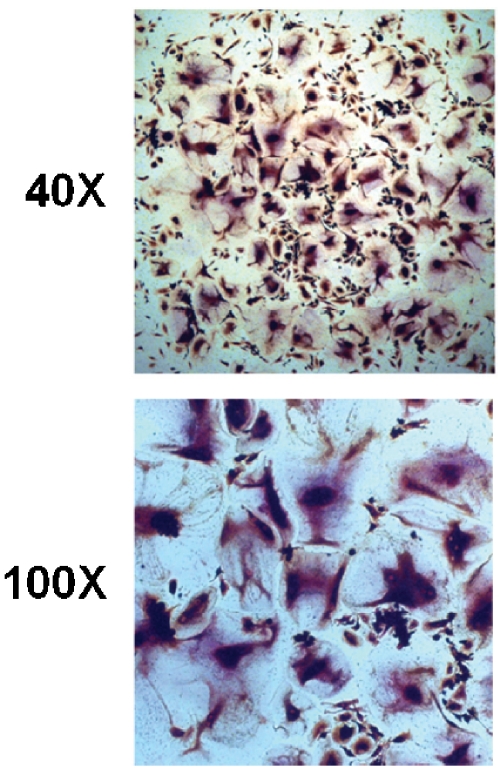
For economic reasons, we wished to employ medium supplemented with murine RANKL for future experiments. Therefore, we first wished to confirm that murine RANKL would support osteoclastogenesis in our simplified protocol. Osteoclast precursors, which had been maintained for two weeks in culture, were seeded into 48-well plates. Cells were cultured in medium containing 100 ng/ml murine RANKL and 12 ng/ml human M-CSF. Osteoclastogenesis was observed within 7-8 days, as in cultures maintained with human RANKL. The cells were stained for TRAP at day 11 and photographed (Figure 3). No qualitative differences from cells cultured with human RANKL were observed.
Figure 3.
Murine RANKL supports the generation of human osteoclasts from cryopreserved bone marrow in a simplified protocol. Human bone marrow macrophages were isolated from whole bone marrow following cryopreservation, and were cultured in the presence of 100 ng/ml recombinant murine RANKL and 12 ng/ml recombinant human M-CSF. Large, multinucleated cells became visible after 8 days and were stained for the presence of TRAP on day 10. Shown are representative micrographs at 40X and 100X magnification.
Bone resorption
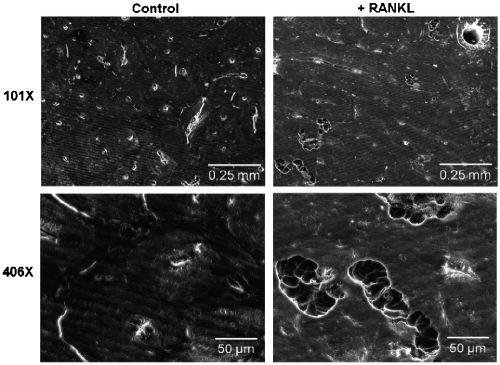
In order to confirm that the osteoclasts produced by our simplified protocol were functional, we employed these cells in a bone resorption assay. A24-well plate containing slices of devitalized bovine cortical bone was seeded with 2 x 105 osteoclast precursor cells. Cultures were maintained in osteoclastogenic medium for 18 days. In areas of the wells adjacent to the bone slices, we observed osteoclast formation within 7-8 days, in accordance with our previous experiments. As expected, these cells were not observed in control wells cultured without RANKL. In order to verify that these osteoclasts were functionally active, we analyzed the bone slices harvested from these cultures on day 18 by SEM. As indicated in Figure 4, resorption pits were observed on slices from cells cultured in the presence of RANKL, but not those from control cultures that lacked RANKL. Hence, the osteoclasts generated in our system were fully functional in their ability to resorb bone.
Figure 4.
Human osteoclasts generated from cryopre-served bone marrow in a simplified protocol exhibit resorptive capability. Human osteoclasts were generated on slices of devitalized bovine cortical bone. Pre -osteoclasts were cultured in the presence of 10 ng/ml recombinant human M-CSF and 100 ng/ml recombinant murine RANKL (right column) or without RANKL (left column) as a control. After 18 days, bone slices were subjected to SEM, which revealed the presence of resorption pits in RANKL-containing wells. Shown are representative micrographs at both 10IX and 406X magnification.
Discussion
We have described a simplified system by which human osteoclasts may be generated in vitro from bone marrow macrophages. These cells were obtained from a commercial source and immediately cryopreserved. Upon thawing, mononuclear cells were isolated by density gradient centrifugation and then plated in tissue culture flasks for 24 hours. Subsequently, nonadherent cells were harvested and plated in medium containing recombinant RANKL and M-CSF. Under these conditions, osteoclast formation was observed within 7 days and confirmed by TRAP staining. The resorptive capability of these cells was confirmed by SEM in similar experiments in which cells had been plated on slices of bovine cortical bone.
Whereas PBMCs are more commonly used for human osteoclastogenesis experiments, we elected to use whole bone marrow as the source material for our system. The use of bone marrow macrophages is desirable from an economical standpoint, as whole bone marrow offers a greater number of cells on a per-dollar basis than PBMCs or bone marrow-sourced sub-populations that are also commercially available. A study by Schilling et al. showed that bone marrow contains more osteoclast precursors per ml than does peripheral blood [17]. Moreover, bone marrow cells may represent a more authentic precursor population than PBMCs. While other investigators have also reported osteoclastogenesis from bone marrow cultures, these protocols typically require 3-4 weeks for osteoclast formation [7, 9–12,19]. As we have confirmed here, less time is required if cultures are supplemented with M-CSF and RANKL [8]. These protocols rely on the entire mononuclear fraction of bone marrow cells or, in some cases, only the cells that remain adherent after an initial incubation period. In contrast, our experiments utilized only the nonadherent cells, which can be considered a more pure osteoclast precursor population, as the bone marrow stromal cells are no longer present. Flanagan and Massey as well as Li et al. have also reported osteoclastogenesis from this population of cells [8, 19]. Lader et al. suggested that osteoclasts generated in co-cultures more closely resemble true osteoclasts, as those generated in the absence of stromal cells express more macrophage-associated integrins [14]. However, these experiments utilized PBMCs as precursors rather than bone marrow cells, and were co-cultured with rodent stromal cell lines. Whether the same phenomenon is true of bone marrow-derived osteoclasts has not been determined. Thus, we attempted to establish physiologically relevant co-cultures that would support osteoclastogenesis without the addition of recombinant RANKL. Bone marrow macrophages were co-cultured with bone marrow stromal cells or commercially available primary osteoblasts under a variety of experimental conditions, but osteoclast differentiation was not observed in these cultures. However, Atkins et al. demonstrated that primary human osteoblasts induce osteoclastogenesis from PBMCs or bone marrow macrophages, using a defined, serum-free medium but not in serumcontaining medium [15].
All of the experiments described here were conducted on cells that had been cryopreserved. This finding confirms similar observations in studies conducted on cryopreserved cells sourced from both PBMCs [6] and bone marrow [8]. The establishment of a cryopreserved stock of cells, along with the ability of these cells to expand in vitro, allows entire sets of assays to be conducted on cells from a single source. In this way, investigators can assay the effects of various compounds on osteoclastogenesis while controlling for differences among individuals. This is important, as it is likely that age, gender, race, and other donor-specific factors may influence the osteoclastogenic potential of a particular bone marrow sample. Conversely, it would be possible to ianvestigate the effects of these factors on osteoclastogenesis by compiling a library of cells sourced from different donors and assaying their osteoclastogenic efficiency in parallel. While it is possible that the osteoclastogenic potential of the cells is reduced by cryopreservation, to our knowledge, this question has not been directly investigated.
Particular attention was paid during this study to an economical use of cells. Consequently, we relied on relatively low cell densities, ranging from 1.7 × 104 to 1 × 105 cells/cm2, for our experiments. Cell densities in other protocols employing bone marrow are typically several fold higher, ranging from as low as approximately 6 x 104 cells/cm2 [8] to 3 × 105 [8, 12, 19] or 5 × 105 cells/cm2 [9, 11]. Schilling et al., in experiments with precursors sourced from buffy coat, demonstrated that the efficiency of osteo-clast differentiation increases with increasing cell density, until a threshold is reached [17]. However, the number of osteoclasts formed at the most efficient density (107 cells/ml) was less than an order of magnitude higher than that at the least efficient density (104 cells/ml). We sought to balance osteoclast formation with an efficient use of cells. The most advantageous density may vary with the needs of individual investigators.
There are several methods for the generation of human osteoclasts in vitro, each of which may have certain advantages or disadvantages. We have combined the most advantageous aspects of other protocols into one simplified approach. This protocol offers the following advantages: an economical source of cells, a simple set-up, a limited number of required medium supplements, and a shortened time frame. We offer this system as a simplified process which can be easily adopted by laboratories lacking prior experience with osteoclast culture.
Acknowledgements
The authors would like to thank Robin D. Foley at the UAB Electron Optics Laboratory for assistance with electron microscopy. This work was supported by NIH grants RO1 CA108585 (JTD), T32 CA075930 (JJC), and RO1 AR47830 (XF).
References
- [1].Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- [2].Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoaprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- [3].Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis- inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feng X, Novack DV, Faccio R, Ory DS, Aya K, Boyer Ml, McHugh KP, Ross FP, Teitelbaum SL. A Glanzmann's mutation in beta 3 integrin specifically impairs osteoclast function. J Clin Invest. 2001;107:1137–1144. doi: 10.1172/JCI12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nardelli B, Zaritskaya L, McAuliffe W, Ni Y, Lincoln C, Cho YH, Birse CE, Halpern W, Ullrich S, Moore PA. Osteostat/tumor necrosis factor superfamily 18 inhibits osteoclastogenesis and is selectively expressed by vascular endothelial cells. Endocrinology. 2006;;147:70–78. doi: 10.1210/en.2005-0518. [DOI] [PubMed] [Google Scholar]
- [6].Susa M, Luong-Nguyen NH, Cappellen D, Zamurovic N, Gamse R. Human primary osteoclasts: in vitro generation and applications as pharmacological and clinical assay. J Transi Med. 2004;2:6. doi: 10.1186/1479-5876-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Quinn JM, Neale S, Fujikawa Y, McGee JO, Athanasou NA. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int. 1998;62:527–531. doi: 10.1007/s002239900473. [DOI] [PubMed] [Google Scholar]
- [8].Flanagan AM, Massey HM. Generating human osteoclasts in vitro from bone marrow and peripheral blood. Methods Mol Med. 2003;80:113–128. doi: 10.1385/1-59259-366-6:113. [DOI] [PubMed] [Google Scholar]
- [9].Takahashi N, Kukita T, MacDonald BR, Bird A, Mundy GR, McManus LM, Miller M, Boyde A, Jones SJ, Roodman GD. Osteoclast-like cells form in long-term human bone marrow but not in peripheral blood cultures. J Clin Invest. 1989;83:543–550. doi: 10.1172/JCI113916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hughes DE, MacDonald BR, Russell RG, Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest. 1989;83:1930–1935. doi: 10.1172/JCI114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heymann D, Gouin F, Guicheux J, Munevar JC, Godard A, Daculsi G. Upmodulation of multinucleated cell formation in long-term human bone marrow cultures by leukaemia inhibitory factor (LIF) Cytokine. 1997;9:46–52. doi: 10.1006/cyto.1996.0134. [DOI] [PubMed] [Google Scholar]
- [12].Sarma U, Flanagan AM. Macrophage colony-stimulating factor induces substantial osteoclast generation and bone resorption in human bone marrow cultures. Blood. 1996;88:2531–2540. [PubMed] [Google Scholar]
- [13].Oreffo RO, Kusec V, Virdi AS, Flanagan AM, Grano M, Zambonin-Zallone A, Triffitt JT. Expression of estrogen receptor-alpha in cells of the osteoclastic lineage. Histochem Cell Biol. 1999;111:125–133. doi: 10.1007/s004180050342. [DOI] [PubMed] [Google Scholar]
- [14].Lader CS, Scopes J, Horton MA, Flanagan AM. Generation of human osteoclasts in stromal cell-free and stromal cell-rich cultures: differences in osteoclast CD11c/CD18 integrin expression. Br J Haematol. 2001;112:430–437. doi: 10.1046/j.1365-2141.2001.02437.x. [DOI] [PubMed] [Google Scholar]
- [15].Atkins GJ, Kostakis P, Welldon KJ, Vincent C, Findlay DM, Zannettino AC. Human trabecular bone-derived osteoblasts support human osteoclast formation in vitro in a defined, serum-free medium. J Cell Physiol. 2005;203:573–582. doi: 10.1002/jcp.20255. [DOI] [PubMed] [Google Scholar]
- [16].Ramalho AC, Couttet P, Baudoin C, Morieux C, Graulet AM, de Vernejoul MC, Cohen-Solal ME. Estradiol and raloxifene decrease the formation of multinucleate cells in human bone marrow cultures. Eur Cytokine Netw. 2002;13:39–45. [PubMed] [Google Scholar]
- [17].Schilling AF, Linhart W, Filke S, Gebauer M, Schinke T, Rueger JM, Amling M. Resorb-ability of bone substitute biomaterials by human osteoclasts. Biomaterials. 2004;25:3963–3972. doi: 10.1016/j.biomaterials.2003.10.079. [DOI] [PubMed] [Google Scholar]
- [18].Sarma U, Edwards M, Motoyoshi K, Flanagan AM. Inhibition of bone resorption by 17beta-estradiol in human bone marrow cultures. J Cell Physiol. 1998;175:99–108. doi: 10.1002/(SICI)1097-4652(199804)175:1<99::AID-JCP11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- [19].Li F, Chung H, Reddy SV, Lu G, Kurihara N, Zhao AZ, Roodman GD. Annexin II stimulates RANKL expression through MAPK. J Bone Miner Res. 2005;20:1161–1167. doi: 10.1359/JBMR.050207. [DOI] [PubMed] [Google Scholar]
- [20].McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]