Figure 7.
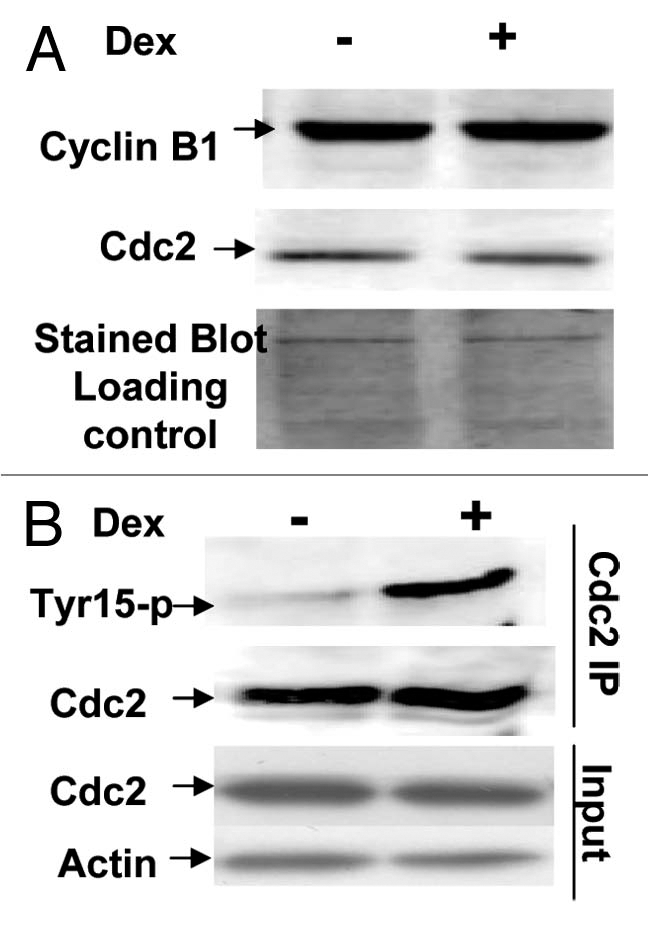
Induction of Wip1 increases the level of inhibitory tyrosine phosphorylation on Cdc2 protein. (A) The levels of nuclear cyclin B and cdc2 proteins are the same between the Wip1 induced and uninduced cells at time of 12 h. The western blot prepared from the nuclear fraction of Wip1 induced (+) and uninduced (−) U2–15 cells at time of 12 h, was blotted by cyclinB and cdc2 antibodies, respectively. There is no detectable difference in both protein levels of Cdc2 and cyclin B between lane (−) and lane (+). Cytoplasmic levels of both proteins were also the same between Wip1 induced and uninduced cells (data not shown) (the stained blot for the control of protein loading). (B) The level of inhibitory tyrosine phosphorylation on Cdc2 is increased when wip1 is induced. The immunoprecipitate from the total soluble protein of uninduced (lane−) and induced (lane+) U2–15 cells at time of 12 h using anti Cdc2 antibody was detected by anti-phosphotyrosine-15 monoclonal antibody. There is an increased level of inhibitory phosphotyrosine on Cdc2 protein in Lane (+). The same blot shown in the top part of (B) was stripped and detected using anti-Cdc2 antibody. There is no detectable difference in Cdc2 protein level between lane (−) and lane (+) (the middle part of B). The IP input control is provided (the bottom part of B).
