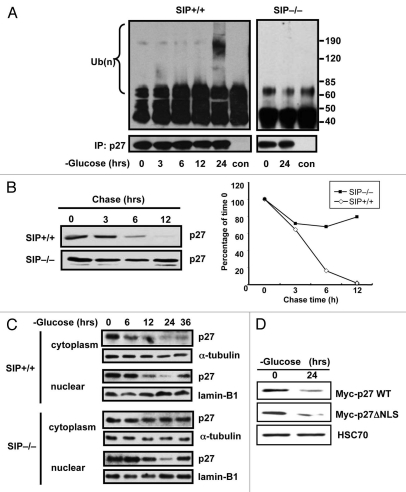
Figure 3.
Glucose limitation induces poly-ubiquitination of cytoplasmic p27 protein. (A) Synchronized wild-type and SIP−/− MEFs were cultured in low glucose media and 10% dialyzed FCS and cell lysates prepared at the indicated times. Endogenous p27 proteins were immunoprecipitated with anti-p27 antibody and the immunoprecipitates analyzed by immunoblotting with anti-ubiquitin or p27 antibodies. (B) Wild-type or SIP−/− MEFs cells in 100 mm dishes were transiently transfected with 3 µg of plasmids producing with Myc-tagged p27 plasmid for 48 h and cells were cultured in glucose free media. After 48 h, cells were treated with 25 µg/ml cycloheximide, were harvested at the indicated times and an equal amount of protein from each lysate was analyzed by immunoblotting using anti-Myc antibody. The degree of p27 expression was quantitated by densitometric analysis with ImageJ software and expressed as a percentage of p27 expression at 0 h. (C) Synchronized wild-type and SIP−/− MEFs were cultured in low glucose media and cell lysates were prepared at the indicated times. Cell lysates were fractionated to cytosolic and nuclear fractions and levels of p27 proteins analyzed by immunoblotting using anti-p27, Lamin B1 and α-tubulin antibodies. (D) NIH3T3 cells stably expressing Myc-tagged wild-type p27 or mutant of nuclear localization signal p27 (ΔNLS) were cultured in media containing 0.1 mM glucose and 10% dialyzed FCS. After 48 h, cell lysates were analyzed by immunoblotting using antibodies specific for Myc and HSC70.