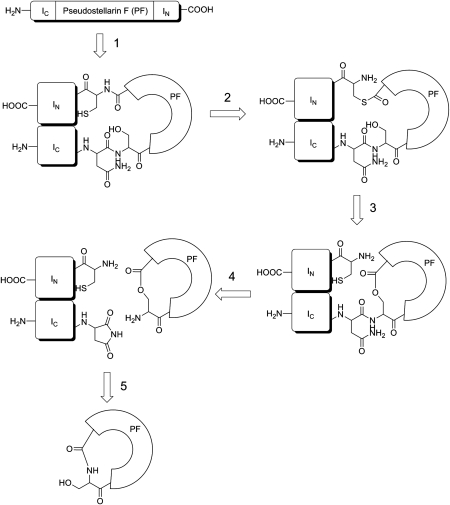
Figure 2.
Mechanism for intein-mediated cyclization of pseudostellarin F The expressed precursor folded to form an active protein ligase (reaction 1); an N-to-S acyl shift was catalyzed to give a thioester intermediate (reaction 2) that underwent transesterification to form a lariat intermediate (reaction 3); Succinimide formation released the cyclic product as a lactone (reaction 4); the final cyclopeptides was generated by an X-to-N acyl shift (reaction 5). The illusion was modified from references [19,40].