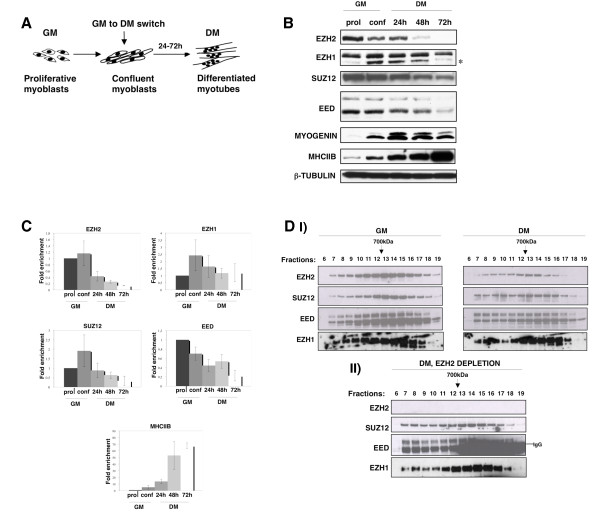
Figure 1.
Dynamics of PRC2-Ezh2 and PRC2-Ezh1 complexes during C2C12 skeletal muscle cell differentiation. (A) Schematic representation of C2C12 skeletal muscle cell differentiation. Proliferative myoblasts at 80% confluency were induced to differentiate for 24-72 h by replacing growth medium (GM) with differentiating medium (DM). (B) Immunoblot of Ezh2, Ezh1, Suz12 and Eed from whole cell extracts of cells cultured as myoblasts in GM (prol = proliferative myoblasts at 50% confluence; conf = 80% confluent myoblasts) or as myotubes in DM (24 h, 48 h and 72 h after differentiation induction). Myogenin and myosin heavy chain IIB (MHCIIB) were used as muscle differentiation controls for the early and late stages of differentiation, respectively. β-Tubulin was used as a loading control. Asterisk indicates EZH1 unspecific band. Different bands of Eed represent the isoforms of this protein. (C) Expression levels of Ezh2, Ezh1, Suz12 and Eed were measured by real-time PCR in myoblasts grown in GM or DM, for 24 h, 48 h or 72 h after induction of differentiation. MHCIIB was used as a muscle differentiation control. The transcription levels were normalised to Gapdh expression and represent the mean of three independent experiments ± SD. Fold enrichment was calculated in comparison to myoblasts in GM. (D) (I) Size exclusion chromatography of nuclear extracts prepared from myoblasts in GM (left panel) or DM (72 h after differentiation induction) (right panel) showing coelution of Ezh2, Suz12, Eed and Ezh1 in high molecular weight fractions. The indicated fractions were analysed by immunoblot. (II) Ezh2 was immunodepleted from extracts prepared from cells cultured in DM (72 h after differentiation induction) and nuclear extract were analysed as described in (I).