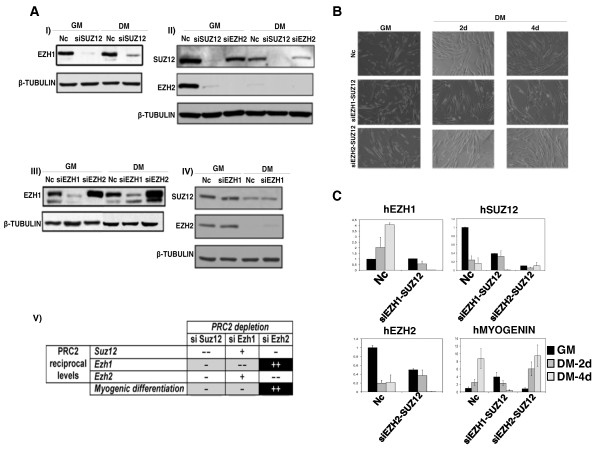
Figure 7.
Ezh1 is the unique PRC2 component required for skeletal muscle differentiation. (A) Immunoblot analysis of Ezh1, Suz12 and Ezh2 levels in whole cell extracts prepared from C2C12 myoblasts cultured in growth medium (GM) and differentiation medium (DM), 48 h after differentiation induction, in Suz12-depleted (I, II), Ezh2-depleted (II, III) and Ezh1-depleted (III, IV) cells. β-Tubulin served as a loading control. (V) Schematic representation of the muscle differentiation phenotypes detected after downregulation of single PRC2 components (Suz12, Ezh1 and Ezh2) and the impact on the protein levels of the remaining members of the complex. PRC2 reciprocal levels: - indicates that protein levels decline; -- indicates that protein levels strongly decline; + indicates that protein levels increase; ++ indicates that protein levels strongly increase. Myogenic differentiation: - indicates a delay in muscle differentiation; ++ indicates an acceleration of muscle differentiation. Phenotypes showing a delay in muscle differentiation are highlighted in grey, while phenotypes showing acceleration are in black. (B) Human myoblasts were transfected with non-targeting small interfering RNA (siRNA) (Nc = negative control) or siRNAs against Ezh1-Suz12 and Ezh2-Suz12. The effect of protein downregulation on cell morphology was analysed in GM (48 h after transfection) and in DM (2 and 4 days after differentiation induction) by phase-contrast microscopy. (C) The efficiency of each siRNA and the expression levels of myogenin (MyoG) were tested by real-time PCR in GM and in DM (2 days and 4 days after differentiation induction) in human myoblasts depleted for specific PRC2 components, as described in (B). The transcription levels were normalised to Gapdh expression and represented as the average of three independent experiments ± SD. Fold enrichment was calculated in comparison to the negative control siRNA in GM.