Table 2. Thermodynamic parameters for the binding of Pb2+ to Tau244–372 (or full-length Tau protein) as determined by ITC at 25.0°C.
| Tau244–372 | K d (µM) | n |
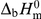 (kcal mol−1) (kcal mol−1) |
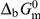 (kcal mol−1) (kcal mol−1) |
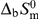 (cal mol−1 K−1) (cal mol−1 K−1) |
| WT | 0.217±0.029 | 1.090±0.003 | −6.78±0.04 | −9.08±0.08 | 7.73±0.39 |
| H330A | 82±48 | 0.59±0.32 | −0.31±0.21 | −5.79±0.36 | 17.7±1.8 |
| H362A | 0.546±0.020 | 0.165±0.047 | −7.85±0.29 | −8.53±0.22 | 2.32±1.72 |
| DM | NB | – | – | – | – |
| TM | 0.286±0.042 | 1.130±0.005 | −6.19±0.05 | −8.92±0.08 | 9.17±0.45 |
| Histidine-less | NB | – | – | – | – |
| Full-length Tau | 0.29±0.16 | 2.72±0.06 | −7.86±0.30 | −8.91±0.32 | 3.55±2.05 |
Thermodynamic parameters, Kd, 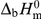 , and n, were determined using a single set of identical sites model. The standard molar binding free energy (
, and n, were determined using a single set of identical sites model. The standard molar binding free energy (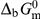 ) and the standard molar binding entropy (
) and the standard molar binding entropy (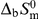 ) for the binding reaction were calculated using Equations 2 and 3 respectively.
) for the binding reaction were calculated using Equations 2 and 3 respectively.
The buffer used was 50 mM Bis-Tris buffer (pH 7.4). Errors shown are standard errors of the mean.
WT, wild-type Tau244–372; DM, double mutant H330A/H362A of Tau244–372; TM, triple mutant H268A/H299A/H329A of Tau244–372; Histidine-less, histidine-less mutant H268A/H299A/H329A/H330A/H362A.
NB, no binding observed in the present conditions.
