Abstract
Despite the growing popularity of the zebrafish model system, the optimal husbandry conditions for this animal are not well defined. The aim of this study was to examine the effect of stocking density on reproductive performance in zebrafish. In this study, undertaken by eight different zebrafish facilities, clutches of at least 200 wild-type zebrafish embryos from a single pairwise mating were produced at each participating institution and subsequently reared according to “in-house protocols” until they were 14 weeks old. Fish were then randomly assigned into treatment groups with balanced sex ratios and densities of 3, 6, or 12 fish/L. After a 1-month acclimation period, fish were spawned in pair crosses every 2 weeks for 3 months, for a total of six spawning dates. The number of viable and nonviable embryos produced in each clutch were counted at 1 day postfertilization. Although there was a great deal of variability in clutch size and percent spawning success among laboratories, there were no significant differences in average clutch size, spawning success, or percent viable among the treatment densities. These data suggest that using stocking densities as high as 12 fish/L does not have a negative impact on performance, when measured by reproductive performance.
Introduction
The zebrafish has become a well-established laboratory animal model because of its many favorable attributes, including optical clarity of the embryo, amenability to genetic manipulation, and tolerance of a wide range of environmental conditions. Interestingly, the same hardy character of the fish that has made them attractive to researchers has also delayed the optimization of husbandry conditions. Some of the most basic husbandry questions remain unanswered, including how many fish to keep in a tank, and how this affects reproductive performance. The study described in this report was organized by the Zebrafish Husbandry Association (ZHA) as a baseline study to answer these questions and provide data on clutch size, fertilization rate, and percent spawning success in different laboratories. These data have great value as a basis for the development of more formal, traditional studies1 and as a basic reference on reproductive performance for the fish research community.
The vast majority of research on fish densities in recirculating aquaculture systems has been on fish species raised for consumption, wherein tank densities are reported in weight/volume measurements and, in many cases, are orders of magnitude greater than current zebrafish laboratory stocking densities. The focus of much of this research is on raising fry and juvenile stage animals to the adult stage for human consumption. For example, recommended stocking densities for recirculating aquaculture systems with aeration but without direct oxygen infusion are between 30 and 40 g/L.2 An average adult zebrafish weighs ∼0.5 g,3,4 so this density recommendation converts to 60–80 zebrafish/L. Because the goals of food production aquaculture and zebrafish laboratory aquaculture are different, these numbers may not be directly relevant to zebrafish facilities. Cage stocking densities for other commonly used vertebrate research animals such as mice and rats are often several fold higher than common laboratory zebrafish stocking densities when compared on a mass-per-volume basis.5 The dramatic environmental and physiological differences between mammals and fish make these comparisons difficult to make and likely inappropriate.
Most zebrafish system manufacturers recommend stocking densities between 6 and 15 fish/L (personal communication). “The Zebrafish Book” suggests housing zebrafish in 10-gallon aquariums at a stocking density of 0.66 fish/L.6 “A Virtual Tour of the Guide for Zebrafish Users” recommends a stocking density of 5 fish/L, but they also report that stocking densities have not been critically tested.7 The eighth edition (2011) of the “Guide for the Care and Use of Laboratory Animals”5 cites Matthews et al.7 and also recommends 5 fish/L with the caveat that this recommendation could change with new research.1 The “Guide for the Care and Use of Laboratory Animals” is the reference book that is used by the Institutional Animal Care and Use Committees and the Association for Assessment and Accreditation of Laboratory Animal Care International when evaluating animal programs.
It is important to note that none of these guidelines is based on published, peer-reviewed data. To this end, we organized and conducted these collaborative experiments to begin to explore the effects of densities on one of the primary indicators of zebrafish condition, reproductive success.
Although the lack of standardization in zebrafish facility management and husbandry makes research in these areas important to the zebrafish community, it also presents challenges for study design and interpretation. Results from rigorously controlled studies conducted at a single institution, although completely valid, may be of only limited value to the larger zebrafish community because of the numerous and considerable differences in environment and management that are likely to exist between facilities. This study was designed as a collaboration between a number of representative zebrafish laboratories displaying a typical wide range in management practices to allow us to make more broad conclusions about the effects of tank density on reproductive performance. An implicit goal here is to provide baseline data that can be used as the basis for future experiments on a single or narrow range of parameters where a tighter control of variables is more feasible and appropriate.
Materials and Methods
Eight zebrafish laboratories from seven different institutions located in North America, Europe, and Australia participated in this study. The identity of the participating laboratories will remain anonymous in this report and will hereafter be referred to as Laboratories 1–8.
Each laboratory followed the same experimental approach, using the wild-type strain most commonly employed in their facility (Table 1). Fry from one pairwise mating that produced at least 200 embryos were raised until 14 weeks postfertilization, at which point they were randomly split into three treatment densities with even sex ratios: 3, 6, and 12 fish/L, rounding to the closest even number of fish so that each tank would have the same number of males and females. Fish were housed in these holding tanks at the treatment densities for the duration of the experiment except when set up for spawning. Six laboratories set up 4 tanks at 3 fish/L, 2 tanks at 6 fish/L, and 2 tanks at 12 fish/L. More tanks were used at 3 fish/L to even out the number of subsamples in each treatment group. Two laboratories, #2 and #3, did not have enough fish to accommodate all eight experimental tanks. Laboratory 2 set up 4 tanks at 3 fish/L, 2 tanks at 6 fish/L, and 1 tank at 12 fish/L and Laboratory 3 set up 3 tanks at 3 fish/L, 2 tanks at 6 fish/L, and 1 tank at 12 fish/L. Each laboratory set up one additional tank stocked at 8 fish/L to be used for mortality replacement in treatment tanks if necessary.
Table 1.
Husbandry Parameters at Each Lab
| Wild type used | Fry feeding regime | Spawning adult feeding regime | Age of visual sexual dimorphism | Age when consistent spawning begins | Mortality rate at 0–1 month | Average adult mortality over 3 months | Fry stocking density | Adult stocking density | System manufacturer and tank size | Breeding cages used for study | H2O exchange rate as tank changes per hour | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab 1 | EK | Hatchfry Encapsulon, AP100 and decapsulated artemia | Zeigler crumble twice/day decapsulated artemia once/day | 3 months | 4 months | 30% | ∼1% | Up to 30 fry/L | 7–10 fish/L | Marine Biotech Z-mod 2L tanks | Techniplast 0.75 L | 3–4 |
| Lab 2 | AB | Hatchfry Encapsulon, AP100, and decapsulated artemia | Zeigler crumble twice/day decapsulated artemia once/day | 3 months | 4 months | 30% | ∼1% | Up to 30 fry/L | 7–10 fish/L | Marine Biotech Z-mod 2L tanks | Techniplast 0.75 L | 3–4 |
| Lab 3 | TuAB | Hatchfry Encapsulon and decapsulated artemia | decapsulated artemia twice/day, Flake (omega one or spirulina, krill, plankton flake) | 2.5 months | 3.5 months | 10–20% | <5% | Up to 15 fry/L | 2–3 fish/L (9 L tanks) | AHAB 1, 2.75, and 9 L tanks | AHAB 1 L | 6 |
| Lab 4 | AB X TL F1 hybrid | Paramecium and/or decapsulated artemia + powered dried food gape size appropriate 3 times/day | Various pelleted food + freeze dried plankton and decapusalted artemia 3 times/day | 3–4 months | 4 months | 15% | <1% | Up to 16 fry/L | 5 fish/L | AHAB 10 and 3L tanks | AHAB 1 L | 6 |
| Lab 5 | AB | Artemia 4 times/day, Liquified mix spirulina and encapsulon 2–3 times/day | Artemia enriched with Selco or spirulina twice/day, Lansey Pellet with spirulina twice/day | ∼2 months | 5 months | 25% | 10% | Up to 20 fry/L | 3–4 fish/L (11 L tanks) | Aquarienbau Schwarz 1.8 and 11 L tanks | 2 L mouse cages | 4 |
| Lab 6 | AB | Sera Micron (ad libitum) Artemia 4 times/day or Proton pellets | pellets 3 times/day decapsulated artemia once/day | 1.5–2 months | 3.5–4 months | 10–20% | <10% | 20–25 fry/L | 5 fish/L | Aquaneering 1, 2, and 9 L tanks | AHAB 1 L | 8 |
| Lab 7 | AB | Nannochloropsis, Hatchfry Encapsulon, paramecia, and decapsulated artemia 3 times/day | Cyclop-eeze 3 times/week, decapsulated artemia once/day, Zeigler crumble once/day | 1.5 months | 2.5–3 months | <10% | Not measured | 30 fry/L reduced to 7 fry/L at day 20 | 8 fish/L | AHAB 1.5, 2.75, and 10 L tanks | AHAB 2 L (filled to 1 L) | 5–6 |
| Lab 8 | AB | Paramecia 3 times/day, INVE proton pellet daily, artemia 2 times/day | dry food once daily (INVE food 6/8 (600 to 1000 μm) and PerlaAq) once daily decapsulated artemia | 3 months | 3.5–4 months | <10% | <1% | 60 fry/500 mL | 4 fish/L | AHAB 1.5, 2.75, and 10 L tanks | AHAB 2 L | 5–6 |
Underlined and italicized text represents conditions that were specifically used in the present study. All other entries apply to general husbandry practices at each institute.
After a 1-month acclimation period at the treatment densities, reproductive performance was assayed by setting up all of the fish in each treatment tank in pairwise matings (one male and one female per breeding cage). Fish were set up in crosses between 1 and 5 pm in the afternoon of a selected day and were allowed to spawn until between 10 am and 12 pm the next day when eggs were collected into Petri dishes and stored at 28.5°C. At 1 day postfertilization (dpf), the numbers of viable and nonviable embryos were counted and recorded for each cross. After crossing, all fish were returned to their original treatment tank. This crossing protocol was repeated every 2 weeks for 3 months, for a total of six spawning dates.
Each laboratory fed treatment tanks proportionally based on the number of fish in each tank. Feed type as well as other husbandry information from each of the eight laboratories is listed in Table 1.
A list of water quality parameters measured is shown in Table 2, including the range and frequency of measure for each parameter at each laboratory.
Table 2.
Water Quality at Each Lab
| |
Disolved O2 (mg/L) |
pH |
Ammonia (ppm) |
Nitrite (ppm) |
Nitrate (ppm) |
Conductivity (us) |
Total hardness (ppm) |
Temperature (°C) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Range | Frequency | Range | Frequency | Range | Frequency | Range | Frequency | Range | Frequency | Range | Frequency | Range | Frequency | Range | |
| Lab 1 | Constant | 5.5–8.3 | Constant | 7.0–7.6 | Weekly | 0 | Weekly | 0 | Weekly | 17–30 | Constant | 920–1100 | Weekly | 120 | Constant | 26–28 |
| Lab 2 | Constant | 5.5–8.3 | Constant | 7.0–7.6 | Weekly | 0 | Weekly | 0 | Weekly | 17–30 | Constant | 920–1100 | Weekly | 120 | Constant | 26–28 |
| Lab 3 | a | a | Constant | 7.3–7.5 | Weekly | 0 | Weekly | 0 | Weekly | 0–50 | Constant | 450–550 | a | a | Constant | 26–30 |
| Lab 4 | a | a | Constant | 6.8–7.4 | Weekly | 0 | Weekly | 0 | Weekly | 0 | Constant | 420–480 | Seldom | 7 | Constant | 27.5–29.5 |
| Lab 5 | Monthly | 6.8–8.4 | Daily | 6.9–7.6 | Weekly | 0–0.25 | Weekly | 0–0.2 | Weekly | 10–40 | Daily | 1000–2300 | Monthly | 126–200 | Constant | 24–27 |
| Lab 6 | Daily | 5–8 | Daily | 6.5–7.5 | 2 × /week | 0–0.1 | Weekly | 0–0.5 | Monthly | 0–50 | Daily | 400–750 | 2 × /week | 5–15 | Daily | 27–28.5 |
| Lab 7 | Daily | 95% | Daily | 7.4 | Daily | 0 | Daily | 0 | Daily | 0 | Daily | 850 | Daily | 120 | Daily | 28.5 |
| Lab 8 | a | a | 2 × /week | 7.2–7.6 | 2 × /week | <0.2 | 2 × /week | <0.2 | 2 × /week | 0–100 | 2 × /week | 400–800 | a | a | Constant | 28–29 |
Not measured.
Statistical analysis
Average clutch size, percent spawning success, and percent viable at 1 dpf were the parameters measured. Average clutch size was defined as the average number of viable embryos produced by one spawning pair at a given density. Percent spawning success was calculated as the total number of successful spawns at a given density divided by the total number of crosses set up at that density, multiplied by 100. A spawning was considered successful if it produced 15 or more viable embryos. Percent viable at 1 dpf was calculated as the total number of viable embryos produced at a given density at 1 dpf divided by the total number of embryos produced at 1 dpf multiplied by 100. Total number of embryos included viable eggs, unfertilized eggs, eggs of poor quality that could not be fertilized, and embryos that did not survive to 1 dpf.
To analyze the data from all institutes on all spawning dates, the means for each parameter at each institute were used, making a given density at each institute the experimental unit for a total of eight experimental units for each treatment. A one-way analysis of variance (ANOVA) was performed to detect significant differences (p < 0.05) between 3, 6, and 12 fish/L for average clutch size, percent spawning success, and percent viable at 1 dpf.
In addition to comparing stocking densities across all laboratories, differences among stocking densities within laboratory were also tested for all three parameters. For within-laboratory comparisons, averages for each individual holding tank on each sample date were used as the experimental unit. A two-way ANOVA was run with laboratory and density as factors. The interaction between laboratory and density was significant (clutch size, p = 0.004; spawning rate, p = 3.7 × 10−7; percent viable, p = 0.001), so we ran the one-way ANOVA described above. A Student's t-test assuming unequal variances with Bonferroni correction was used to detect significant differences between the three parameters within each institute (adjusted p < 0.0167). Significance testing for percent spawning success and percent viable was performed on arcsine-transformed data to improve normality.8 Significance testing before and after arcsine transformation was different for only two comparisons, both of which are reported.
Results
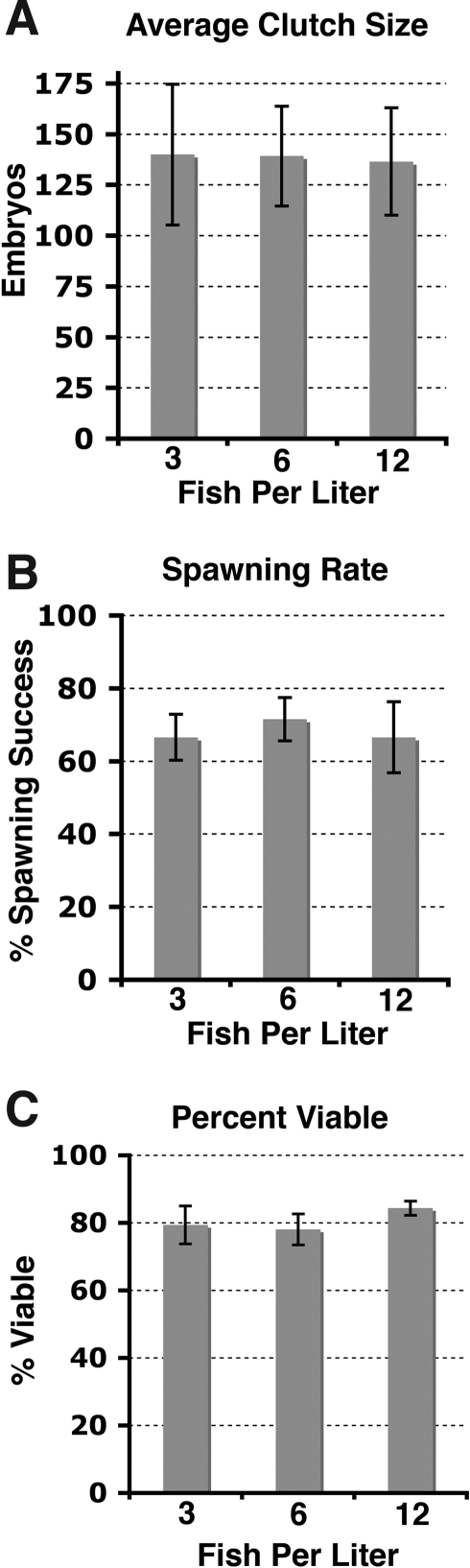
After over 2000 individual pairwise crosses at eight different zebrafish laboratories, no significant difference was found among treatment densities (3, 6, and 12 fish/L) in average clutch size (Fig. 1A), percent spawning success (Fig. 1B), or percent viability (Fig. 1C) when compared across all laboratories.
FIG. 1.
Average clutch size (A), spawning rate (B), and percent viable at 1 day postfertilization (C) for individual pairwise crosses of zebrafish housed at three different stocking densities: 3, 6, and 12 fish/L. Data were averaged from eight zebrafish laboratories collected on six spawning dates. Vertical bars represent the standard error of the mean. No significant differences were detected among any of the three densities for any parameter using one-way analysis of variance.
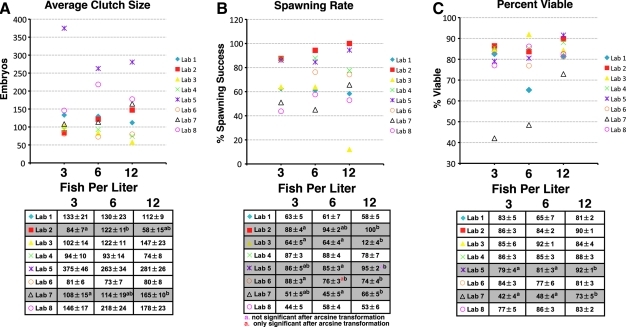
Average clutch size at each institute was highly variable and ranged from 49 embryos (Fig. 2A, Lab 3, 12 fish/L) to 378 embryos (Fig. 2A, Lab 5, 3 fish/L) with Laboratory 5 recording six instances of clutches larger than 1000 embryos. Laboratories 2 and 7 showed significant within-facility differences in average clutch size among treatment densities (Fig. 2A).
FIG. 2.
Scatter plots of average clutch size (A), spawning rate (B), and percent viable at 1 day postfertilization (C) for eight different zebrafish laboratories. Each point represents the average from six spawning dates of pairwise crosses of zebrafish housed at three different stocking densities: 3, 6, and 12 fish/L. The tables below the plots show the means (±standard error of the mean) for each density at each lab. Laboratories that had significant differences among any of the three tank densities are highlighted in gray. Student's t-test with Bonferroni correction (p < 0.0167) was used to detect differences among treatment densities within each institute. Values that share letters are not significantly different.
Spawning rate was also highly variable, ranging from 12% (Lab 3, 12 fish/L) to 100% (Lab 2, 12 fish/L), with the majority of facilities falling between 40% and 95% (Fig. 2B). Laboratories 2, 3, 5, 6, and 7 showed some significant within-facility differences in spawning rate among the three densities (Fig. 2B).
Percent viability was less variable among facilities, with all showing viability rates between 71% and 92% with the exception of Laboratory 7 (Fig. 2C). Both Laboratories 5 and 7 showed significant within-facility differences in percent viable among the three treatment densities (Fig. 2C).
The results of all crosses at all institutes are presented in Supplementary Figure S1; available online at www.liebertonline.com/zeb.
Discussion
As the use of zebrafish as an experimental model continues to increase, it is important to broaden our understanding of the optimal husbandry conditions required for zebrafish culture. Husbandry optimization will not only lead to improved efficiency for zebrafish laboratories, but published husbandry data will contribute to more useful, reasonable, and informed regulation. In this study we show that stocking densities as high as 12 fish/L do not negatively affect reproductive performance. This result is striking in light of the fact that it is more than twice that of presently published guideline of 5 fish/L.6,7 These data also have implications for space efficiency, because holding densities can be effectively doubled without impacting reproductive performance. For example, if laboratories were to switch from housing fish at 5 fish/L to housing fish at 12 fish/L they could more than double the number of animals housed in a given space, without having to increase the size of the facility. A density where reproductive performance and general health are compromised certainly exists, but we have not reached that density in this study when reproductive performance is used as an indicator.
Another important finding is that clutch size and percent spawning success were highly variable among laboratories, likely because of a lack of standardization. Some laboratories reported improved performance at low densities, whereas other laboratories reported improved performance at higher densities (Fig. 2). This variation in the effect of density on reproductive performance at different institutes illustrates the challenge of establishing husbandry standards based on the results of a single well-controlled experiment conducted at one institution. In this study, the overall trend was that there was no difference between the three treatment densities (Fig. 1) when averaged across all laboratories.
The average clutch size for Laboratory 5 was more than double the average clutch size from all other institutes, with six spawns that produced >1000 embryos each. Because clutch size and percent spawning success are important to a laboratory's productivity, further studies to exactly determine why Laboratory 5 had such superior reproductive performance would be of great benefit to the zebrafish community.
Table 1 highlights some of the differences between the laboratories that participated in this study. These differences are likely to be responsible for at least some of the demonstrated variability in reproductive performance, but as this table is far from complete, it would be impossible to make conclusions about correlations between husbandry parameters found in the table and reproductive performance. Some key differences between laboratories that were not recorded include the genetic maintenance strategy used to maintain broodstock used in the experiment and the volume of food offered, both of which could be as responsible for the reproductive variability as any of the factors measured in Table 1.
In many fish species, an increase in stress leads to impaired reproductive performance (reviewed by Schreck et al.9 and Barton and Iwana10), but the relationship between stocking density and stress remains unclear. Growth rates have been shown to increase with increasing stocking densities in juvenile11 and adult Arctic charr,12 and in European sea bass, specific growth rate has been shown to increase and food conversion ratio to decrease with increasing stocking densities,13 suggesting that in some species high stocking densities may improve growth performance when compared with lower stocking densities. In other fish species, increasing stocking density leads to decreased growth rates,14,15 but the densities used in those experiments are much higher than those used in the present study. An experiment examining cortisol levels in zebrafish exposed to crowding stress found that crowding fish at 40 fish/L in a 4-L tank did not show significant increases in cortisol compared with control fish; but, when crowded at the same density in a 76-L tank a significant increase in cortisol was seen, likely because of the difference in precrowding densities.16 In the present study, we did not have any direct measures of the stress response, but if we consider reproductive performance as an indirect measure of the stress response, there was no difference at any of the densities tested.
Future studies are needed to determine the maximum stocking density for laboratory-reared zebrafish and to understand why such variability exists in reproductive performance among laboratories. Further investigations specifically designed to examine the effects of other husbandry parameters such as feed type, water quality, and genetic strain on reproductive performance are also needed. Data collected from such efforts will be undoubtedly useful for improving the efficiency of zebrafish facility management practices and for developing performance-based standards for zebrafish care and use.
Supplementary Material
Acknowledgments
The authors express gratitude to Artie McCollum for his help with organizing the ZHA working group on reproduction and spawning and to Benoit Chambaron for his participation in the original working group discussions. Additionally, the authors thank Dede Greenstein for help in running the two-way ANOVA. The authors also thank all the zebrafish husbandry staff members who assisted with zebrafish care at all laboratories participating in this study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture. 2007;269:1–20. [Google Scholar]
- 2.Losordo TM. Masser MP. Rakocy JE. Recirculating Aquaculture Tank Production Systems, a Review of Component Options. Stoneville, MS: Southern Regional Aquaculture Center; 1999. Publication No. 453. [Google Scholar]
- 3.Petrie-Hanson L. Romano CL. Mackey RB. Khosravi P. Hohn CM. Boyle CR. Evaluation of zebrafish Danio rerio as a model for enteric septicemia of catfish (ESC) J Aquat Anim Health. 2007;19:151–158. doi: 10.1577/H06-026.1. [DOI] [PubMed] [Google Scholar]
- 4.Cui Z. Samuel-Shaker D. Watral V. Kent ML. Attenuated Mycobacterium marinum protects zebrafish against mycobacteriosis. J Fish Dis. 2010;33:371–375. doi: 10.1111/j.1365-2761.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press; 2010. [Google Scholar]
- 6.Westerfield M. The Zebrafish Book. 4th. Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- 7.Matthews M. Trevarrow B. Matthews J. A virtual tour of the guide for zebrafish users. Lab Anim. 2002;31:34–40. doi: 10.1038/5000140. [DOI] [PubMed] [Google Scholar]
- 8.Draper NR. Smith H. Applied Regression Analysis. Second. New York: Wiley and Sons; 1981. [Google Scholar]
- 9.Schreck CB. Contreras-Sanchez W. Fitzpatrick MS. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture. 2001;197:3–24. [Google Scholar]
- 10.Barton BA. Iwana GK. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis. 1991;1:3–26. [Google Scholar]
- 11.Wallace JC. Kolbeinshavn AG. Reinsnes TG. The effects of stocking density on early growth in Arctic Char, Salvelinus alpinus (L.) Aquaculture. 1988;73:101–110. [Google Scholar]
- 12.Jorgensen EH. Christiansen JS. Jobling M. Effects of stocking density on food intake, growth performance and oxygen consumption in Arctic charr (Salvelinus alpinus) Aquaculture. 1993;110:191–204. [Google Scholar]
- 13.Papoutsoglou SE. Tziha G. Vrettos X. Athanasiou A. Effects of stocking density on behavior and growth rate in European sea bass (Dicentrarchus labrax) juveniles reared in a closed circulated system. Aquacult Eng. 1998;18:135–144. [Google Scholar]
- 14.Montero D. Izquierdo MS. Tort L. Robaina L. Vergara JM. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol Biochem. 1999;20:53–60. [Google Scholar]
- 15.Ellis T. North B. Scott AP. Bromage NR. Porter M. Gadd D. The relationships between stocking density and welfare in farmed rainbow trout. J Fish Biol. 2002;61:493–531. [Google Scholar]
- 16.Ramsey JM. Feist GW. Varga ZM. Westerfield M. Kent M. Schreck CB. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture. 2006;258:565–574. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.