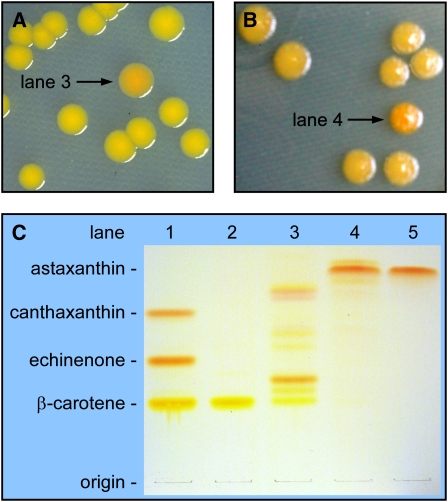
Figure 2.
Color Complementation Screening of an A. aestivalis cDNA Library Enabled the Identification of cDNAs That Encode the Enzymes Needed to Convert β-Carotene into Astaxanthin.
(A) An A. aestivalis cDNA library was introduced into a strain of E. coli that had been engineered to produce β-carotene (cells contained plasmid pAC-BETA). Cultures inoculated with the rare orange colonies (e.g., that indicated with an arrow) selected from among a multitude of yellow colonies were found to produce a complex mixture of carotenoids (lane 3 of [C]) with 4-hydroxy and/or 3,4-didehydro-β-rings (Cunningham and Gantt, 2005).
(B) A second screening of the A. aestivalis cDNA library was performed in E. coli engineered to produce those pigments found in orange colonies selected in the initial screen (cells contained plasmid pAC-BETA-CBFD1/2, constructed by inserting the two cDNAs recovered from library plasmids selected in the initial screen, cbfd1 and cbfd2, into pAC-BETA). Colonies a darker orange to red in color (e.g., indicated with an arrow) were selected for analysis.
(C) Reverse-phase TLC separation of pigments extracted from E. coli containing the plasmids listed below (lanes 1 to 4) and of a synthetic astaxanthin standard (lane 5). Lane 1, pAC-BETA + pHPK (contains an H. pluvialis cDNA that encodes an enzyme that converts β-carotene into echinenone and canthaxanthin); lane 2, pAC-BETA; lane 3, pAC-BETA and pCBFD1 (a cDNA library plasmid recovered from the orange colony indicated with an arrow in [A]); lane 4, pAC-BETA-CBFD1/2 and pHBFD1 (a cDNA library plasmid recovered from that colony indicated with an arrow in [B]); lane 5, astaxanthin standard. Note: The apparent colors of carotenoids on TLC plates are very much affected by the concentrations of the pigments, with higher concentrations of yellow carotenoids appearing more orange to red in color.