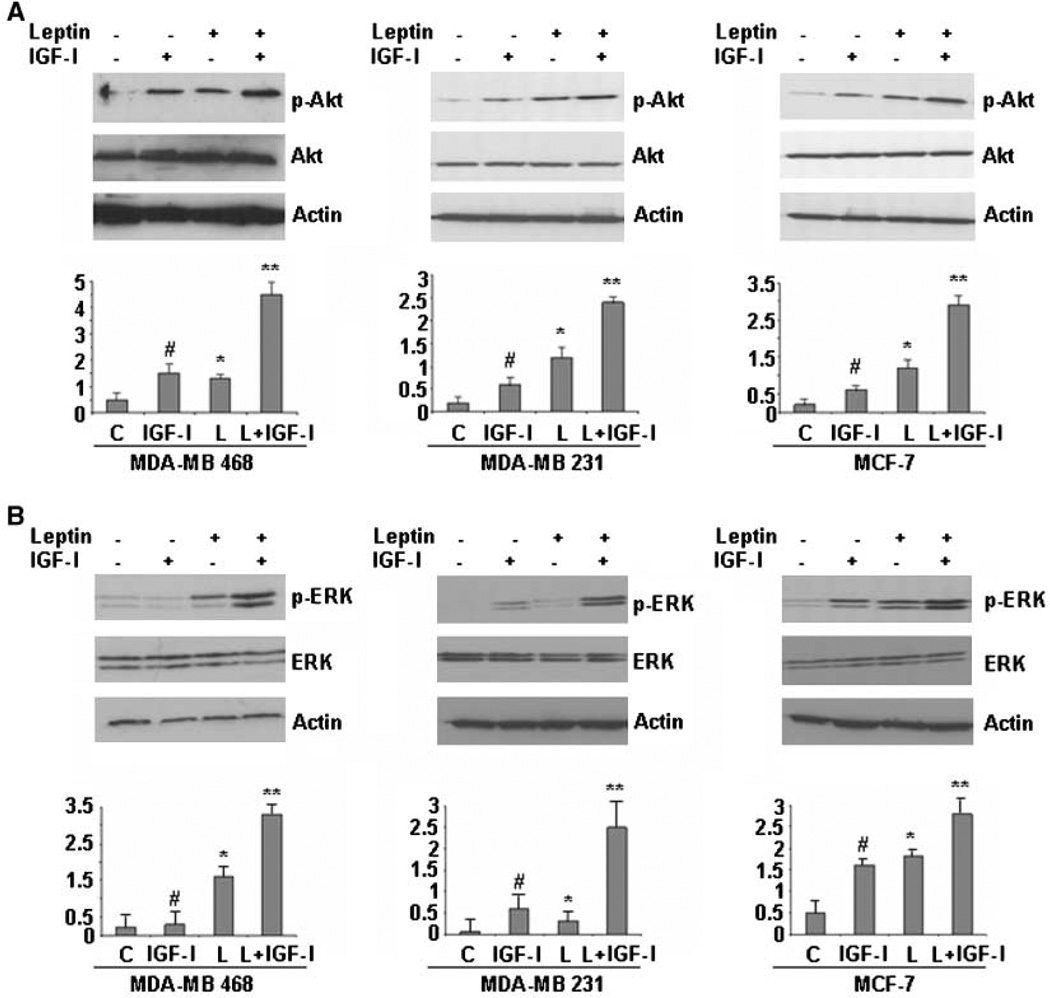
Figure 3.
Combined treatment with leptin and IGF-I leads to a synergistic increase in phosphorylation of both Akt and ERK in breast cancer cells. MDA-MB-468, MDA-MB-231, and MCF-7 cells were serum starved for 24 h, followed by treatment with 100 ng/mL leptin, 100 ng/mL IGF-I alone, and the combination for 30 min. Total protein was isolated from MDA-MB-468, MDA-MB-231, and MCF-7 cells and equal amounts of proteins were subjected to immunoblot analysis with specific antibodies against phospho-Akt (p-Akt) and Akt (A) or phospho-ERK (p-ERK) and ERK (B). Increased phosphorylation of Akt was observed in MDA-MB-468, MDA-MB-231, and MCF-7 cells treated with leptin or IGF-I, whereas combined treatment induced further increase in Akt phosphorylation. Leptin and IGF-I treatment increased phosphorylation of ERK, whereas combined treatment induced a synergistic increase in ERK phosphorylation. The membranes were reblotted with ERK, Akt, and anti-actin antibodies as control. Representative blots of multiple independent experiments. The representative histogram is the densitometric analysis of the Western blot signals showing fold increase in levels of phospho-Akt and phospho-ERK with respect to total Akt and ERK. #, P < 0.01, compared with untreated controls; *, P < 0.05, compared with untreated controls; **, P < 0.01, compared with leptin-treated cells.