Abstract
The first committed biosynthetic step toward clavulanic acid, the clinically-important β-lactamase inhibitor, is catalyzed by the thiamin diphosphate (ThDP)-dependent enzyme N2-(2-carboxyethyl)arginine synthase (CEAS). This protein carries out a unique reaction among ThDP-dependent processes in which a C–N bond is formed, and an electrophilic acryloyl–thiazolium intermediate of ThDP is proposed to be involved, unlike the nucleophilic enamine species typically generated by this class of enzymes. Here we present evidence for the existence of the putative acryloyl adduct, and report the unexpected observation of a long-wavelength chromophore (λ = 433 nm), which we attribute to this enzyme bound species. Chemical models were synthesized that both confirm its expected absorption (λ = 310–320 nm), and exclude self-condensation and intramolecular imine formation with the cofactor as its cause. Circular dichroism experiments and others discount charge transfer as a likely explanation for the ~120 nm red shift of the chromophore (~25 kcal). Examples are well-known of charged molecules that exhibit significantly red-shifted UV-visible spectra compared to their neutral forms as, for example, polyene cations and dyes such as indigo and the cyanines. Rhodopsin is the classic biochemical example where the protein (opsin)-bound protonated Schiff base of retinal displays a remarkable range of red-shifted absorptions modulated by the protein environment. Similar tuning of the chromophoric behavior of the enzyme-bound CEAS acryloyl•ThDP species may be occurring.
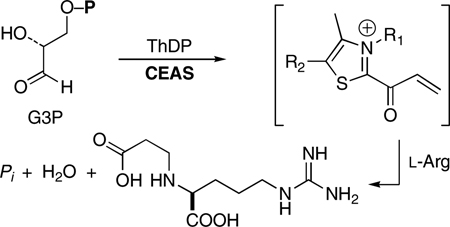
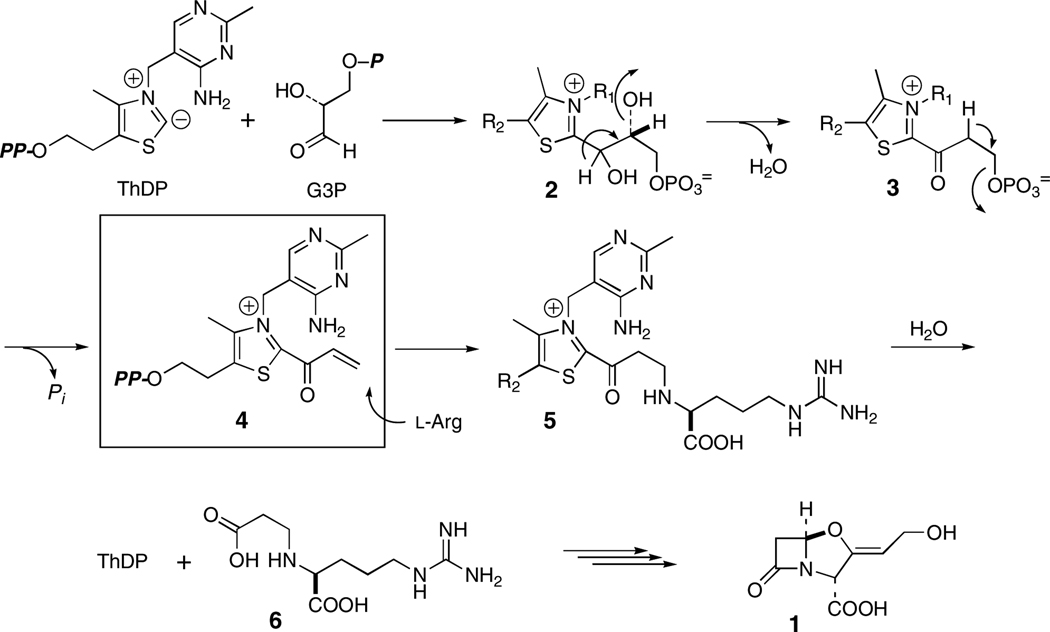
With the rise in resistance to penicillins and cephalosporins, the β-lactamase inhibitor clavulanic acid (1) has grown in importance for the treatment of bacterial infections1. Several mechanistically intriguing reactions take place in the biosynthesis of 1,2 the first of which is mediated by N2-(2-carboxyethyl)arginine synthase (CEAS). The primary metabolites d-glyceraldehyde-3-phosphate (G3P) and l-arginine are recruited by this enzyme and reacted in a thiamine diphosphate (ThDP)-dependent internal redox process to yield N2-(2-carboxyethyl)arginine (6, CEA; Scheme 1).3 The mechanism shown in Scheme 1 was proposed in accord with extensive isotopic labeling and stereochemical experiments.3 A key feature of this mechanism was the suggested intermediacy of an acryloyl–ThDP adduct 4. The observation of overall retention of configuration at the G3P β-carbon is consistent with a β-elimination/addition process proceeding by way of this highly electrophilic4 partner for reaction with the incoming l-arginine. This is a unique reaction cycle among ThDP-dependent enzymes where typically intermediates formed are nucleophilic rather than electrophilic, and N–C bond formation has not been seen previously. In this Communication we demonstrate the formation of the acryloyl species 4 and report the observation of an unexpectedly long-wavelength chromophore, which owes to the presence of the acryloyl intermediate.
Scheme 1.
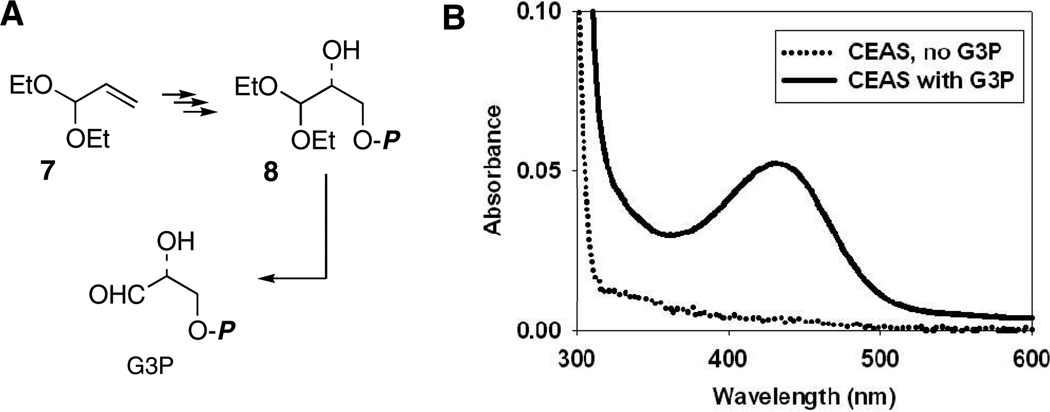
Recombinant CEAS3 was purified to near homogeneity on a large scale using two-phase aqueous separation5, followed by chromatographic steps involving ion exchange and particle size to remove aggregated protein (in preparation). Synthesis of the co-substrate G3P in high optical purity began with acrolein diethylacetal (7) and proceeded according to the literature6 to the diethylacetal of G3P (8, Fig. 1A). Removal of unwanted salts and unreacted organic starting materials and byproducts was achieved by chromatography on microcrystalline cellulose followed by a short C-18 column eluted with water. Analysis of an intermediate alcohol as its Mosher ester showed an enantiomeric purity of >95%. Dowex-50 deprotection of the acetal afforded G3P, which could be quantified in solution by assay with G3P dehydrogenase.7
Figure 1.
A, Synthesis of G3P. B, Spectroscopic observation of the CEAS intermediate at 25 °C, pH 7.8 (CEAS ± G3P).
It was anticipated that incubation of CEAS with G3P alone would generate the acryloyl–ThDP intermediate 4, which, it was hoped, could be dislodged from the protein under acidic conditions as precedented for other enzymes of this family.8 Failure of this approach was not surprising given the intrinsic reactivity of the acryloyl–ThDP adduct under the conditions of the experiment. Turnover of G3P, however, was detected at pH 7.8 by phosphate release9 relative to controls. Expecting the concomitant hydrolytic release of free acrylate, which, while not volatile at this pH, is prone to β-addition, perhaps with CEAS itself, and polymerization. The incubation mixture was lyophilized and separately derivatized with p-nitrobenzylchloride10 or t-butyldimethylsilylchloride11 in DMF. Isolations of the corresponding esters of acrylic acid were coincident with authentic standards by TLC and HPLC, and their distinctive vinyl resonance patterns were observed by 1H-NMR spectroscopy. Multiple turnovers of G3P to acrylate were found, and controls without CEAS gave no spontaneous generation of acrylate.
Quite unexpected during these experiments, however, was the observation of a yellow chromophore (λmax= 433 nm, εM = ~4,000 M−1cm−1, Fig. 1B) thought to arise from the acryloyl intermediate. Addition of sodium dodecylsulfate (SDS, 0.1% w/v) to mildly denature the protein destroyed the chromophore suggesting that its formation is intimately tied to the native state of the protein and not a released byproduct of reaction. Lastly, extended incubation consumed all G3P with loss of the chromophore, but it was restored by addition of fresh substrate. The cycle could be repeated several times.
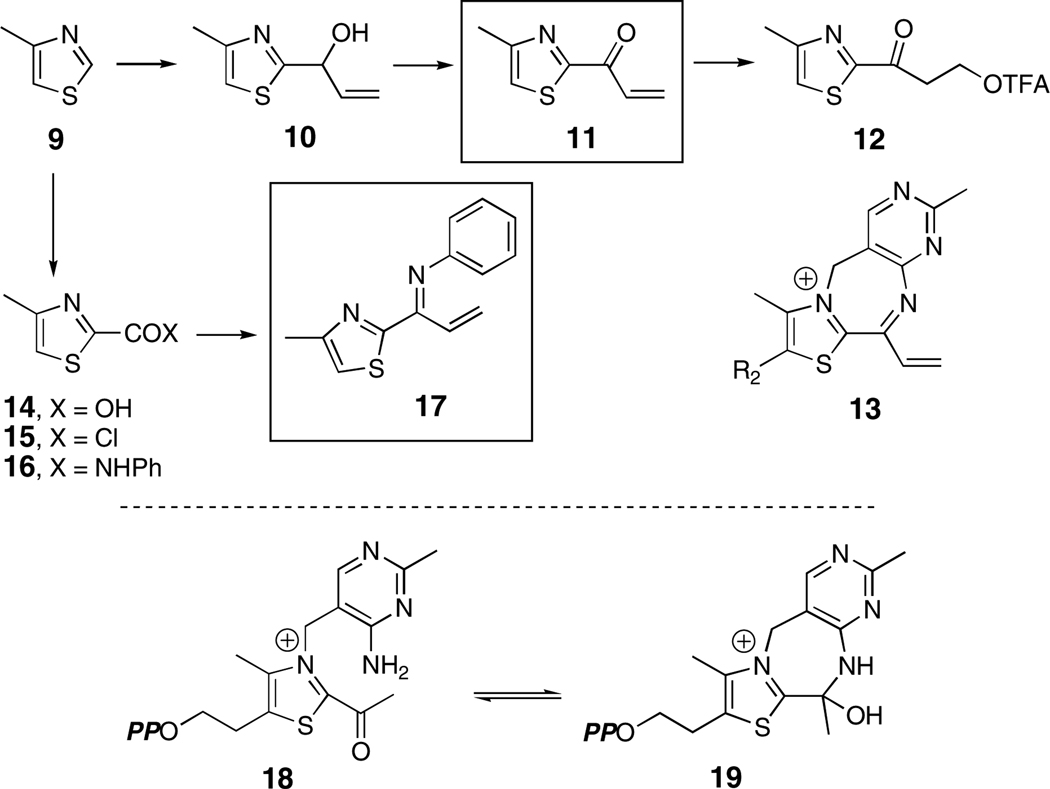
Models of intermediates proposed to be bound to ThDP-dependent enzymes have been synthesized over the years,12–16 and comparison of their UV-visible behavior allows confident prediction of the λmax for acryloyl-ThDP (4) at 310–320 nm in water, a far shorter wavelength than observed for the CEAS intermediate. To confirm this expectation, a synthetic model of 4 was prepared, 2-acryloyl-4-methyl thiazole (11, Scheme 2). 4-Methylthiazole (9) was lithiated at −78 °C with n-BuLi and reacted with acrolein.12 The sensitive allylic alcohol 10 could be oxidized to 11 in 87% yield with the Dess-Martin periodinane.17 Its lowest energy absorption band in acetonitrile (λmax = 316 nm, εM = 7,150 M−1 cm−1) was fully within expectations. Addition of trifluoroacetic acid (TFA) allowed measurement of the transient protonated species, blue-shifted in accord with the literature (λmax = 312 nm),12,15 before rapid conversion to the TFA addition product 12.
Scheme 2.
The high electrophilicity of the acryloyl-ThDP intermediate 4 raised the possibility that intramolecular imine formation with the aminopyrimidine portion of the cofactor could occur to extend the conjugation of the chromophore (13, Scheme 2) and thereby account for its 433 nm absorption. This condensation notwithstanding, reversible hydrolysis can be readily visualized to give acrylate isolated above. Formation of a seven-membered ring was expected to be disfavored, particularly one containing three double bonds. A careful study by Gruys et al. of a related system, acetyl-ThDP (18, Scheme 2) showed that, while imine formation could not be detected, cyclization to the carbinolamine (19, Scheme 2) was clearly evident.12 To examine the UV-spectroscopic behavior of such a possible conjugated π-system, a second model, N-(1-(4-methylthiazol-2-yl)allylidene)benzenamine (17, Scheme 2) was constructed. Once again 4-methylthiazole (9) was lithiated and carbonated to carboxylic acid 14.18 Reaction of the corresponding acid chloride 15 proceeded simply to the anilide 16, which underwent Von Braun reaction19 to the imidoylchloride and Stille coupling20 to the desired through-conjugated model 17. Its UV spectrum in acetonitrile of both the neutral [(λmax = 311 nm, εM = 6,250; 360 nm (sh), εM = 2,440)] and its protonated form (λmax = 377 nm, εM = 12, 600) fell considerably short of that of the acryloyl intermediate on CEAS.
Charge-transfer (CT) phenomena are often invoked to explain bathochromic shifts seen in protein chromophores, and, indeed, they have been proposed for other ThDP-dependent enzymes.21 Three lines of evidence, however, discount CT as a probable cause for the appearance of the 433 nm band in CEAS. First, the ~120 nm (~25 kcal/mol) difference between model compounds and the observed intermediate is unusually large to be rationalized by charge-transfer.22 Second, features in the circular dichroism (CD) spectrum are frequently, although not always, seen as a consequence of CT.21 No changes in the CD spectrum of CEAS were detected upon formation of the chromophore. Third, the appearance of CT bands is typically altered by changes in solvent composition or temperature. This behavior was not observed.
We have isolated acrylic acid from the first half-reaction of CEAS supporting the proposed existence of the acryloyl–ThDP adduct 4 (Scheme 1). The thiazolium ring of this reactive intermediate bears a formal positive charge irrespective of pH. There are well-known examples of charged molecules that exhibit significantly red-shifted UV-visible spectra compared to their neutral forms as, for example, polyene cations23 and dyes such as indigo and the cyanines.24 Rhodopsin is the classic biochemical example where the protein (opsin)-bound protonated Schiff base (PSB) of retinal displays a remarkable range of red-shifted absorptions modulated by the protein environment and a highly conjugated protonated iminium ion. Extensive dipole and electrostatic effects imparted by the protein are thought to “tune” a collective Stark effect for each rhodopsin chromophore.25 The extent to which a similar model can be applied to CEAS will be addressed in due course.
Supplementary Material
Acknowledgment
We thank Dr. J. M. McFadden and K. A. Moshos for advice with synthetic procedures, and we are grateful to the National Institutes of Health for financial support (AI 014937).
Footnotes
Supporting Information Available. Syntheses of model compounds 11 and 17 are described and the acrylate isolation. This material is available free of charge via the Internet at (http://pubs.acs.org/instruct/jacsat.pdf).
References
- 1.Elander RP. Appl, Microbiol. Biotechnol. 2003;61:385–392. doi: 10.1007/s00253-003-1274-y. [DOI] [PubMed] [Google Scholar]
- 2.Townsend CA. Curr. Opin. Chem. Biol. 2002;6:583–589. doi: 10.1016/s1367-5931(02)00392-7. [DOI] [PubMed] [Google Scholar]
- 3.Khaleeli N, Li RF, Townsend CA. J. Amer. Chem. Soc. 1999;121:9223–9224. and refs cited. [Google Scholar]
- 4.Evans DA, Fandrick KR, Song HJ. J. Amer. Chem. Soc. 2005;127:8942–8943. doi: 10.1021/ja052433d. [DOI] [PubMed] [Google Scholar]
- 5.Engel S, Barak Z, Chipman DM, Merchuk JC. J. Chromatogr. B Biomed. Sci. Appl. 2000;743:281–286. doi: 10.1016/s0378-4347(00)00053-0. [DOI] [PubMed] [Google Scholar]
- 6.Pederson RL, Liu KKC, Rutan JF, Chen L, Wong CH. J. Org. Chem. 1990;55:4897–4901. [Google Scholar]
- 7.Sigma-Aldrich, Technical Bulletin [Google Scholar]
- 8.Tittmann K, Golbik R, Uhlemann K, Khailova L, Schneider G, Patel M, Jordan F, Chipman DM, Duggleby RG, Hubner G. Biochemistry. 2003;42:7885–7891. doi: 10.1021/bi034465o. [DOI] [PubMed] [Google Scholar]
- 9.Webb MR. Proc. Nat'l Acad. Sci, USA. 1992;89:4884–4887. doi: 10.1073/pnas.89.11.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li RF, Stapon A, Blanchfield JT, Townsend CA. J. Amer. Chem. Soc. 2000;122:9296–9297. [Google Scholar]
- 11.Mawhinney TP, Madson MA. J. Org. Chem. 1982;47:3336–3339. [Google Scholar]
- 12.Gruys KJ, Halkides CJ, Frey PA. Biochemistry. 1987;26:7575–7585. doi: 10.1021/bi00398a007. [DOI] [PubMed] [Google Scholar]
- 13.Daigo K, Reed LJ. J. Amer. Chem. Soc. 1962;84:659–662. [Google Scholar]
- 14.Oka Y, Kishimot S, Hirano H. Chem. Pharm. Bull. 1970;18:527–533. [Google Scholar]
- 15.Lienhard GE. J. Amer. Chem. Soc. 1966;88:5642–5649. doi: 10.1021/ja00969a017. [DOI] [PubMed] [Google Scholar]
- 16.White FG, Ingraham LL. J. Amer. Chem. Soc. 1962;84:3109–3111. [Google Scholar]
- 17.Ireland RE, Liu LB. J. Org. Chem. 1993;58:2899–2899. [Google Scholar]
- 18.Iversen PE. Acta Chem. Scand. 1968;22:694–695. [Google Scholar]
- 19.Vaughan WR, Carlson RD. J. Amer. Chem. Soc. 1961;84:769–774. [Google Scholar]
- 20.Kosugi M, Koshiba M, Atoh A, Sano H, Migita T. Bull. Chem. Soc., Jpn. 1985;59:677–679. [Google Scholar]
- 21.Nemeria N, Baykal A, Joseph E, Zhang S, Yan Y, Furey W, Jordan F. Biochemistry. 2004;43:6565–6575. doi: 10.1021/bi049549r. [DOI] [PubMed] [Google Scholar]
- 22.Mataga N, Kubota T. Molecular Interactions and Electronic Spectra. New York: Marcel Dekker Inc.; 1970. pp. 371–484. [Google Scholar]
- 23.Sorensen TS. J. Amer. Chem. Soc. 1965;87:5075–5084. [Google Scholar]
- 24.Hamer FM. The Cyanine Dyes and Related Compounds. In: Weissberger A, editor. The Chemistry of Heterocyclic Compounds. New York: John Wiley & Sons; 1969. [Google Scholar]
- 25.Kochendoerfer GG, Lin SW, Sakmar TP, Mathies RA. Trends Biochem. Sci. 1999;24:300–305. doi: 10.1016/s0968-0004(99)01432-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.