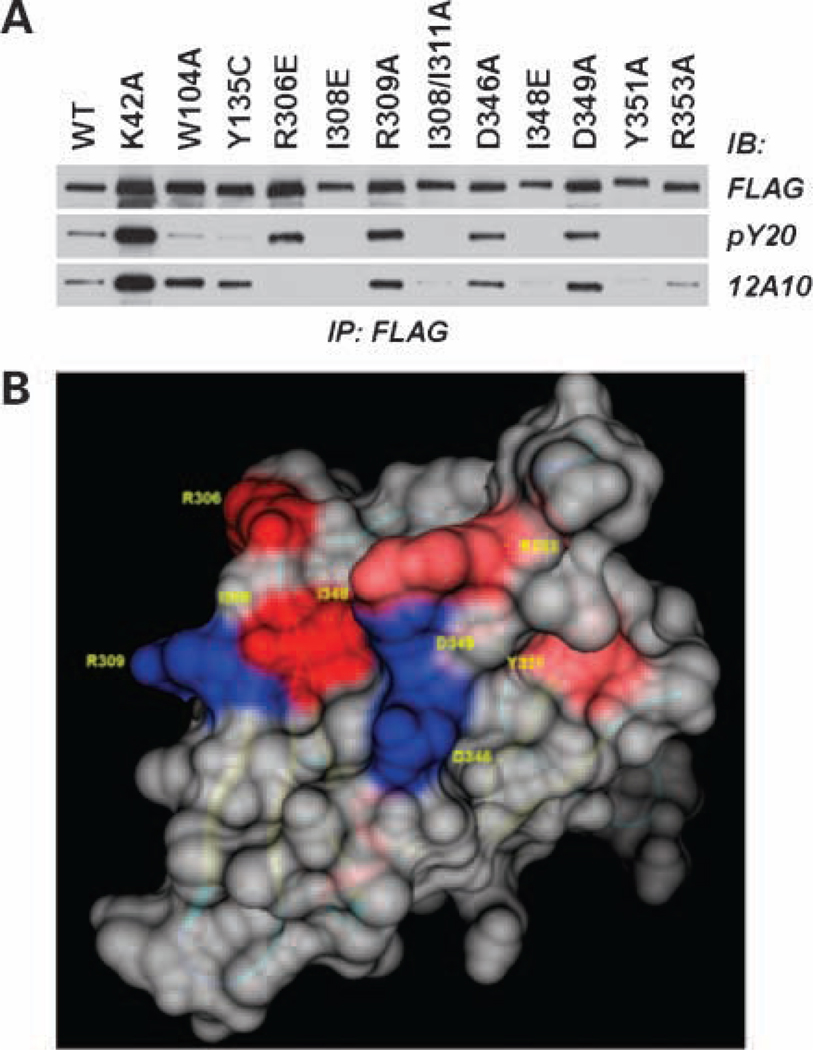
Figure 2.
The mAb 12A10 epitope maps to a surface on the F3 module of the Pyk2 FERM domain. SF767 cells were transfected with FLAG-tagged Pyk2 wild-type (WT) or the indicated FLAG-tagged Pyk2 variant. Pyk2 was immunoprecipitated (IP) with anti-FLAG mAb and immunoblotted (IB) with anti-phosphotyrosine mAb pY20 or mAb 12A10. Antiphosphotyrosine blot was stripped and reprobed with anti-FLAG. B, space fill model of the Pyk2 FERM F3 module highlighting the β5C-α1C surface. Substitution of residues colored blue did not affect mAb 12A10 binding, whereas substitution of residues shaded red abolished or reduced mAb 12A10 binding.