Figure 7. The α subunit of RNAP is acetylated on K297 and K298.
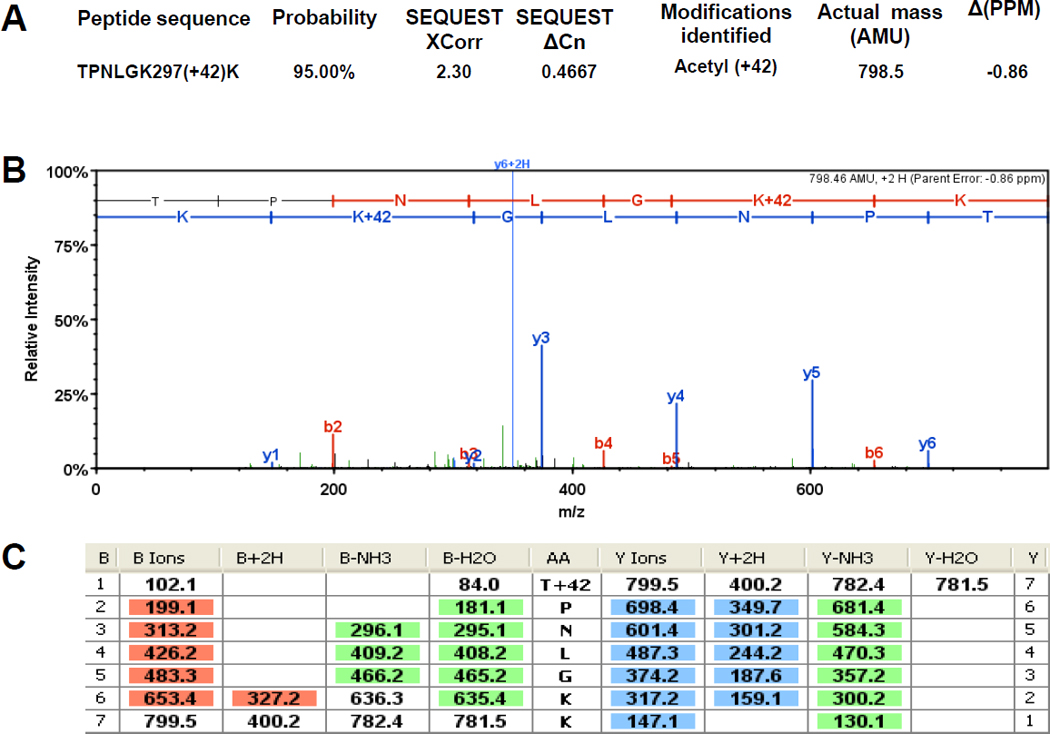
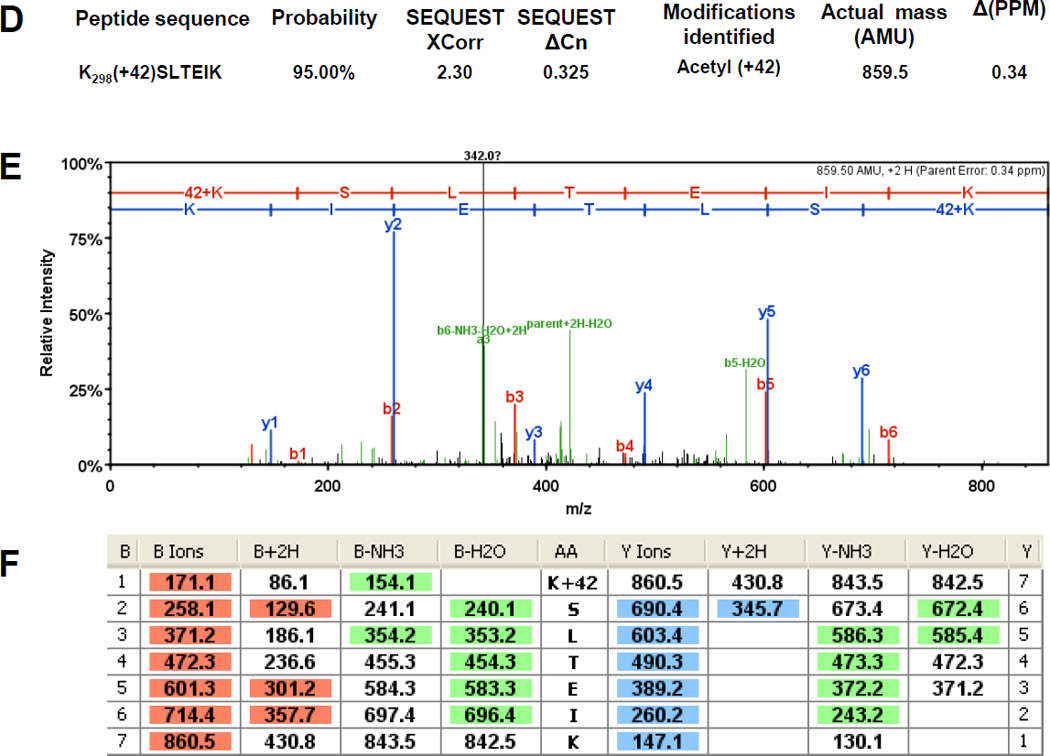
Immunoprecipitated subunits of RNAP were separated by SDS-PAGE. The α bands were excised and tryptically digested as described previously (Chi et al., 2010). The resulting peptides were analyzed in a LTQ OrbitrapXL™ mass spectrometer as described in the Materials and Methods. The double charged acetyllysine-modified peptides TPNLGK297(+42)K and K298(+42)SLTEIK were detected as mass peaks of m/z=400.237 and m/z=430.7582, respectively, in the digested α sample. A and D) The Xcorr and ΔCn scores, the actual peptide masses and mass deviations of the acetylated peptides. B and E) The corresponding CID MS/MS spectra. C and F) Complete b and y fragment ion series for these peptides.
