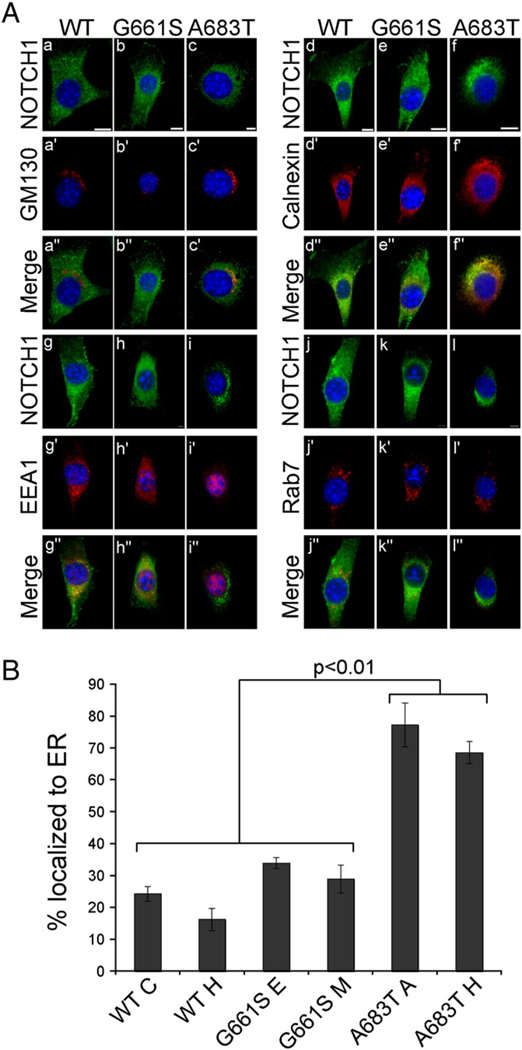
Fig. 3.
The LVOT-associated mutant NOTCH1A683T exhibits improper intracellular trafficking and localization to the endoplasmic reticulum (ER). A. The subcellular location of rNOTCH1 receptors in stable NIH 3T3 cells was assayed by immunofluorescence. Costaining of NOTCH1 with the Golgi-marker GM130 (a–c) did not reveal co-localization in cells expressing rNOTCH1WT (a–a”), rNOTCH1G661S (b–b”), or rNOTCH1A683T (c–c”). Costaining of Notch1 with ER-marker calnexin (d–f) revealed strong co-localization of the mutant rNOTCH1A683T protein with calnexin in the majority of cells observed. Costaining of NOTCH1 with early endosome marker EEA1 (g–i) revealed some co-localization although no significant differences were observed. Similarly, some co-localization with late endosome marker Rab7 was observed, although the degree of co-localization was similar among all cell lines (j–l). Scale bars are 10 µm. B. Two stable lines expressing wild-type or mutant NOTCH1 were co-stained for ER-marker calnexin and NOTCH1 (anti-HA) as above. The co-localization of NOTCH1 and calnexin was assessed in 100 cells, and the percent of cells exhibiting NOTCH1 localization in the ER is shown. In cells expressing rNOTCH1A683T, co-staining reveals a significant increase in localization of mutant protein to the ER (p<.001). In addition, ER localization is slightly lower in cell line WT H compared to lines expressing NOTCH1G661S (p <.05). % ER localization is calculated from three independent experiments. Values were analyzed by ANOVA followed by Bonferroni post hoc.