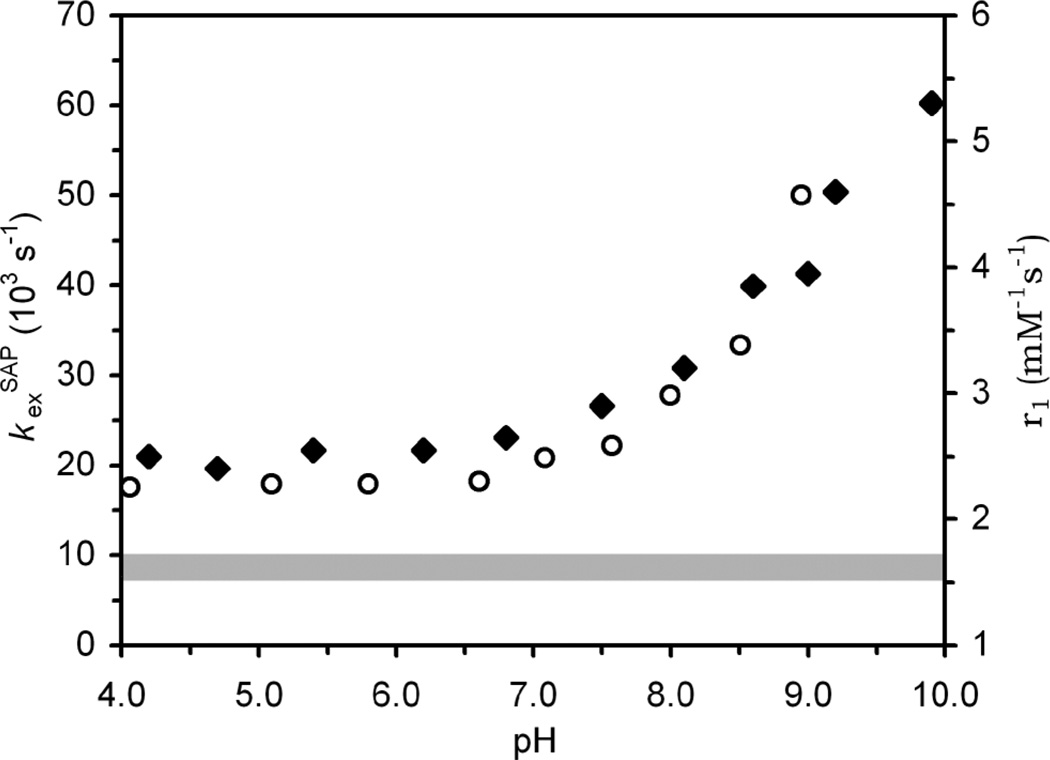
Fig. 2.
The observed exchange rate constant, kexobs, determined for the SAP isomer of EuDOTAMfromCEST experiments (open circles) and the relaxivity (20MHz, 25 °C) of GdDOTAM (closed diamonds) plotted as a function of pH. The grey area represents the probable range of whole water exchange rate constants for the SAP isomer in these chelates as determined from values in Table 1.