Fig 6.
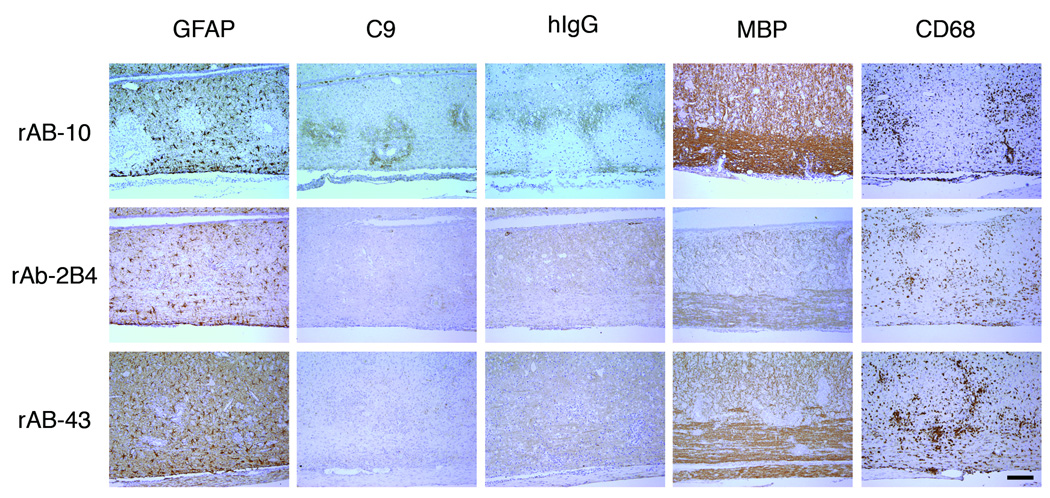
AQP4-specific CSF rAb-10 induced prototypic NMO pathology in guinea pig MBP72–85-induced EAE rats. The rows are arranged by NMO CSF rAb, and the columns are ordered by immunohistochemical stain. In rats injected with rAb-10, glial fibrillary acidic protein (GFAP) immunohistochemistry revealed prominent perivascular loss of astrocyte cell bodies and processes. Staining for complement C9 (C9) and human Ig (hIg) demonstrated significant deposits of perivascular complement protein and human Ig deposition beyond the zone of perivascular astrocyte depletion. Intravenous transfer of negative control measles virus-specific rAb-2B4 or human AQP4-specific rAb-43 produced no additional immunopathology. Myelin basic (MBP) immunohistochemistry demonstrated some perivascular myelin vacuolization in rAb-10-treated animals, but myelin remained largely intact. There was no difference in the extent of CNS tissue macrophage infiltration (CD68) induced by rAbs-10, -43, and -2B4. Scale bar: 200 µM.