Abstract
We report supravalvular aortic stenosis in a 12 year old patient who presented with mental retardation, malformed teeth, broad lower lips, pectus carinatum, clinodactyly, kyphoscoliosis with symptoms of shortness of breath. On examination presence of better volume pulse in right radial artery with ejection systolic murmur best heard in right 2nd intercostal space were noted. Patient was diagnosed as having William's syndrome with investigations demonstrating Supravalvular aortic stenosis with a gradient of 170 mm Hg.
Keywords: Supravalvular aortic stenosis, William's syndrome, elastin
A 12 year old boy presented with exertional dyspnoea since early childhood. On examination he had mental retardation, malformed teeth, broad lower lips, pectus carinatum, clinodactyly and kyphoscoliosis. The right radial pulse was better felt than the left. An ejection systolic murmur was best heard in the right 2nd intercostal space. The patient was diagnosed as having William's syndrome. Investigations showed supravalvular aortic stenosis with a gradient of 170 mmHg. Figure 1 shows pigtail catheter in the left ventricle with opacification of aortic sinuses, coronary vessels, ascending aorta with arch, arch vessels and descending aorta. Labelled structures are right coronary artery (RCA) and left main coronary artery (LMCA). The patient was operated by double-pericardial patch repair of the supra-valvular portion of the aorta with no residual gradient.
Figure 1.
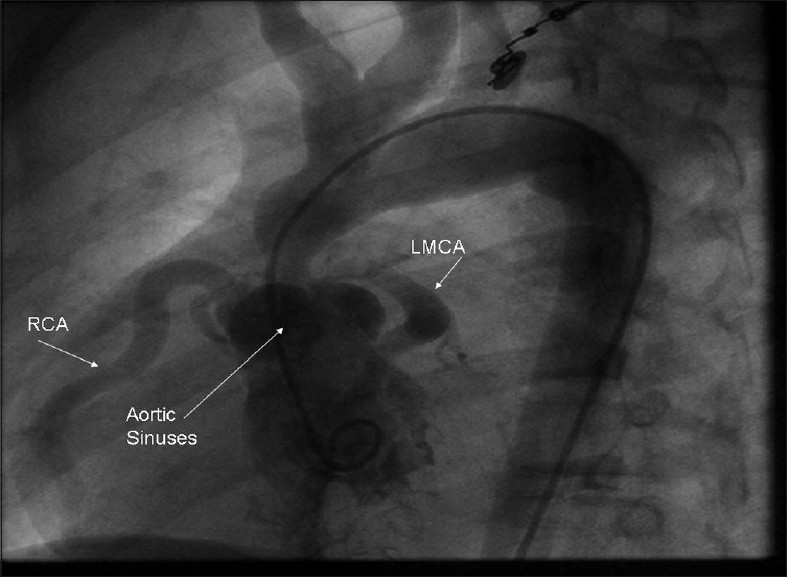
Left Ventricular Cine angiography picture demonstrating clearly the Supravalvular aortic stenosis in a 12 year old patient. Figure shows pigtail catheter in left ventricle with opacification of aortic sinuses, coronary vessels, ascending aorta with arch, arch vessels and descending aorta. Labelled structures are RCA (right coronary artery), LMCA (left main coronary artery)
Photograph of William's syndrome [Figure 2]. Williams–Beuren syndrome is a multisystem disorder caused by deletion of the Williams–Beuren syndrome chromosome region, spanning 1.5 million to 1.8 million base pairs containing 26–28 genes.[1] This syndrome was named after J.C.P. Williams in 1961. In 1962, Alois J. Beuren and associates[2] pointed to the characteristic “elfin facies” and, later, to the possibility of an association with pulmonary artery (PA) branch stenosis. The real frequency of the syndrome is unknown, although it is estimated to be 1 in 10,000–50,000 live births.[3] The involved include cardiovascular system dental, endocrine, gastrointestinal, genitourinary, musculoskeletal and ophthalmic systems. There can be developmental delays with a low intelligence quotient (I.Q.), and characteristic facies are associated with descriptions as cute or pixielike, with a flat nasal bridge, short upturned nose, periorbital puffiness, long philtrum and delicate chin; whereas older patients have slightly coarse features, with full lips, a wide smile and a full nasal tip.
Figure 2.
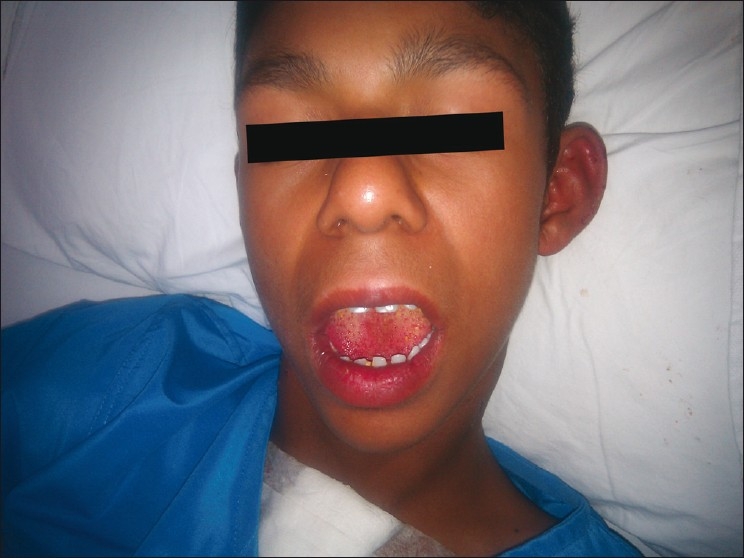
Photograph of child showing characteristic features of William's syndrome
The cardiovascular system is frequently involved. The findings include supravalvular aortic stenosis, peripheral pulmonary artery stenosis, hypertension (renovascular cause is occasionally seen), mitral valve prolapse and ventricular septal defect. Very rarely, it is associated with strokes and sudden death. Supravalvular aortic stenosis and sinotubular junction stenosis are rare outside the setting of Williams–Beuren syndrome, except in familial supravalvular aortic stenosis syndrome. It occurs in approximately 70% of the patients affected with this syndrome.[4] The arterial narrowing is due to thickening of the vascular media from smooth muscle overgrowth and can lead to stenosis of medium and large arteries. The arterial narrowing is also known to occur in numerous locations, including aortic arch, the descending aorta and the pulmonary, coronary, renal, mesenteric and intracranial arteries. An increased carotid artery intima-media thickness consistent with generalized elastin arteriopathy is present in all cases.
The myxomatous degeneration of the aortic or mitral valve leaflets, or both, occur in up to 20% of the patients.[5] Stenosis or occlusion of the coronary ostia can occur in the absence of supravalvular aortic stenosis. Cardiovascular complications are the major cause of death in patients with Williams-Beuren syndrome. One study showed a cardiovascular-associated mortality of 25–100-times than among the controls.[6] The risk factors of sudden death include administration of general anesthesia in cases of biventricular outflow obstruction, biventricular hypertrophy or stenosis or occlusion of coronary ostia.
The diagnosis of this syndrome is suspected because of clinical manifestations and fluorescence in situ hybridization (FISH) involving elastin gene (ELN)-specific probes, establishing the diagnosis of Williams–Beuren syndrome by showing the presence of a single ELN allele rather than two alleles. Diagnosis can also be established by means of microsatellite marker analysis, multiple ligation- dependent probe amplification, quantitative polymerase chain reaction assay or array comparative genomic hybridization.[1]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Pober BR. Wiiliams-Beuren Syndrome. N Engl J Med. 2010;362:239–52. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 2.Beuren AJ, Alpitz J, Harmjanz D. Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation. 1962;26:1235–40. doi: 10.1161/01.cir.26.6.1235. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg F. Williams Syndrome. Pediatrics. 1989;84:922–3. [PubMed] [Google Scholar]
- 4.Pober ER, Johnson M, Urban Z. Mechanism and treatment of cardiovascular disease m Williams-Beuren syndrome. J Clin Invest. 2008;118:1606–15. doi: 10.1172/JCI35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eronen M, Peippo M, Hiippala A, Raatikka M, Arvio M, Johansson R, et al. Cardiovascular manifestations in 75 patients with Wiliams syndrome. J Med Genet. 2002;39:554–8. doi: 10.1136/jmg.39.8.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wesel A, Gravehorst V, Buchhorn R, Aosch A, Partsch CJ, Pankau R. Risk of sudden death in the Williams- Beuren syndrome. Am J Med Genet A. 2004;127A:234–7. doi: 10.1002/ajmg.a.30012. [DOI] [PubMed] [Google Scholar]


