Abstract
The Golgi factory receives custom glycosylates and dispatches its cargo to the correct cellular locations. The process requires importing donor substrates, moving the cargo, and recycling machinery. Correctly glycosylated cargo reflects the Golgi's quality and efficiency. Genetic disorders in the specific equipment (enzymes), donors (nucleotide sugar transporters), or equipment recycling/reorganization components (COG, SEC, golgins) can all affect glycosylation. Dozens of human glycosylation disorders fit these categories. Many other genes, with or without familiar names, well-annotated pedigrees, or likely homologies will join the ranks of glycosylation disorders. Their broad and unpredictable case-by-case phenotypes cross the traditional medical specialty boundaries. The gene functions in patients may be elusive, but their common feature may include altered glycosylation that provide clues to Golgi function. This article focuses on a group of human disorders that affect protein or lipid glycosylation. Readers may find it useful to generalize some of these patient-based, translational observations to their own research.
The Golgi glycosylates and sorts intracellular protein and lipid cargos. Impaired performance by mutated Golgi resident proteins creates severe and highly variable pathologies.
Cargo sorting and glycosylation are the major jobs of the Golgi apparatus. Because 1%–2% of the translated genome affects glycan (sugar chain) biosynthesis and/or binding, it is not surprising that humans have mutations in genes involved in glycan synthesis covering known pathways (Eklund and Freeze 2006). Here we focus on glycosylation disorders mostly discovered within the last 10–12 years (Lowe 2005; Freeze 2006; Jaeken and Matthijs 2007; Clement et al. 2008; Coman et al. 2008; Foulquier 2009; Freeze and Schachter 2009; Guillard et al. 2009). Many disorders that result from biosynthetic defects in the endoplasmic reticulum (ER) also require mention, but the focus remains on those that affect Golgi composition, structure, and homeostasis. The glycan biosynthetic pathways are described briefly here; other articles cover them in more detail.
Human glycosylation disorders assume many guises and their phenotypic expression in model systems and humans is not easy to predict based on cell biology alone; they often assault multiple organ systems. Recent meetings identified areas of consensus and controversy in the Golgi field (Donaldson and McPherson 2009; Emr et al. 2009). Add an assurance that many Golgi-related genetic disorders will be identified in the near future. Discoveries will confirm, extend, and modify our current concepts of Golgi function with new hypotheses, controversies, and, in time, consensus. An understanding of glycosylation will benefit basic scientists in many biological disciplines, but more importantly for patients, it may suggest disease markers and potential therapies.
OVERVIEW OF GLYCOSYLATION PATHWAYS
There are seven major ER-Golgi glycan biosynthetic pathways each defined by the nature of the sugar-protein or sugar-lipid bond. Human disorders occur in each of them. By far, the majority is in the N-glycosylation pathway (GlcNAc-Asn), especially those defects found in the ER. Protein O-glycosylation is more diverse. Ser/Thr residues are linked to glycans through N-acetylgalactosamine, xylose, mannose, or fucose (GalNAc, Xyl, Man, and Fuc, respectively). Each of these has pathway-specific glycosyltransferases that extend the chains. In many cases, terminal sugars are added by more promiscuous transferases that service different pathways (Stanley and Cummings 2009). Glycosphingolipids link glucose (Glc) to membrane-embedded ceramide and the glycan chain is then extended using pathway specific enzymes. Glycophosphatidylinositol (GPI) anchors are initially made in the ER transferred to protein and remodeled in the Golgi. Both glycosphingolipids and GPI anchors are enriched in detergent-insoluble, cholesterol-containing lipid rafts initially assembled in the Golgi and later found on the plasma membrane (Kinoshita et al. 2008; Fujita et al. 2009; Westerlund and Slotte 2009). Recently, defects have been identified in glycosphingolipid glycosylation (SIAT9-CDG, Amish infantile epilepsy) and two defects in glycosylphosphatidylinositol anchor biosynthesis (PIGM-CDG, and PIGV-CDG, glycosylphosphatidylinositol deficiency). A common feature of all Golgi glycosylation pathways is that nucleotide sugar donors must be transported into the Golgi using a series of transporters with different sugar preferences. Not surprisingly, defects in these carriers can affect multiple glycosylation pathways. Table 1 summarizes the ER-Golgi pathway glycans and their general functions.
Table 1.
Classification of the major glycan–protein linkages
| Glycan type | Linkage | Typical proteins | Cell type |
|---|---|---|---|
| N-linked | GlcNAc-β-Asn | Cell surface receptors and secreted proteins | All cell types |
| O-linked | GalNAc-α-Ser/Thr | Secreted and cell surface mucins | Cell surface, gastrointestinal and reproductive tracts |
| O-linked | Man-α-Ser/Thr | α-Dystroglycan | Muscle tissue, nerve cells |
| O-linked | Xyl-β-Ser | Chondroitin/dermatan sulfate & heparan sulfate/heparin | Extracellular matrix, cartilage |
ER LOCATION
Monosaccharide substrates are activated through a series of sugar specific pathways involving kinases, epimerases, and mutases that eventually generate multiple high-energy nucleotide sugar donors (Freeze and Elbein 2009) that can be used directly when acceptor proteins, lipids, or glycans face the cytoplasm. When they face the Golgi lumen, the activated donors require protein-mediated transport into the Golgi.
N-linked glycosylation begins in the ER by a step-wise assembly of a 14-sugar glycan Glc3Man9GlcNAc2 on a PP-dolichol precursor. Initially, the half-completed precursor (Man5 GlcNAc2-PP-dolichol) faces the cytoplasm, but it flips to the luminal side using a flippase. The glycan is completed therein using Dol-P-Man and Dol-P-Glc before oligosaccharyl transferase directs en bloc transfer of the completed glycan to the polypeptide chain emerging from the ribosome into the ER lumen. The protein-bound glycan is then trimmed. Two outermost glucose residues are removed quickly and the third is used as a quality control assurance check. Following its permanent removal, a single Man unit is cleaved clearing the protein for movement to the Golgi. This brief description harbors 18 distinct human glycosylation disorders even prior to the traffic reaching the Golgi (Freeze 2006; Jaeken and Matthijs 2007; Haeuptle and Hennet 2009).
Specific chaperones in the ER help escort proteins into the Golgi and genetic disorders have been found in a few of these as well. One, called COSMC, escorts an O-GalNAc pathway-specific β-galactosyltransferase to its site of action in the Golgi (Ju et al. 2008). A combined loss of coagulation factors V and VIII results from mutations in a transmembrane mannose-binding lectin in the ER-Golgi intermediate compartment (ERGIC-53), and multiple coagulation factor deficiency 2 (MCFD2) its soluble calcium-binding protein partner (Spreafico and Peyvandi 2009; Nishio et al. 2010).
O-fucosylation of selected proteins that carry tandem EGF modules such as Notch or others with thrombospondin type I repeats occurs in the ER, and may have both chaperone and glycan initiating roles (Leonhard-Melief and Haltiwanger 2010; Takeuchi and Haltiwanger 2010). The different protein modules select which of two fucosyltransferases to use and which glycan extensions occur in the Golgi.
Glycosphingolipid synthesis begins in the ER with the synthesis of GlcβCer and all subsequent extensions occur in the Golgi. Mannose-rich GPI-anchors are also constructed in the ER where they are transferred to proteins. Their lipid remodeling may be a signal for their export to the Golgi (Bonnon et al. 2010; Rivier et al. 2010).
GENETIC DISORDERS IN THE MACHINERY
We define the glycosylation machinery as those proteins that participate in the assembly of glycans of one or more pathways. Like most other glycosylation disorders, these are nearly all autosomal recessive. The defective genes are divided by pathway and listed in Table 2. Selected ones are highlighted below.
Table 2.
Golgi-related glycosylation disorders
| Gene function | Gene | OMIM | Clinical presentation | |
|---|---|---|---|---|
| N-glycan disorders | ||||
| MGAT2-CDG (CDG-IIa)a | N-acetylglucosaminyltransferase II | MGAT2 | 212066 | Mental retardation, dysmorphism, seizures |
| I-cell disease | GlcNAc-1-P transferase | GNPTA | 252500 | Severe developmental abnormalities |
| Multiple pathway disorders | ||||
| SLC35C1-CDG (CDG-IIc)a | GDP-fucose transporter | FUCT1 | 266265 | Severe psychomotor retardation, hypotonia, elevated peripheral neutrophils |
| B4GALT1-CDG (CDG-IId)a | β1,4-galactosyltransferase | B4GALT1 | 607091 | Hypotonia, spontaneous hemorrhage, Dandy–Walker malformation |
| COG7-CDG (CDG-IIe)a | Conserved oligomeric Golgi subunit 7 | COG7 | 608779 | Early death, dysmorphism, hypotonia, seizures, hepatomegaly, recurrent infections, cardiac failure, excessive skin |
| SLC35A1-CDG (CDG-IIf)a | CMP-sialic acid transporter | SLC35A1 | 605634 | Normal transferrin, thrombocytopaenia, abnormal platelet glycoproteins |
| COG1-CDG (CDG-IIh)a | Conserved oligomeric Golgi subunit 1 | COG1 | 606973 | Mild MR, hypotonia, growth retardation, progressive microcephaly, hepatosplenomegaly |
| COG8-CDG | Conserved oligomeric Golgi subunit 8 | COG8 | 611182 | MR, hypotonia, encephalopathy |
| COG5-CDG | Conserved oligomeric Golgi subunit 5 | COG5 | — | Moderate mental retardation with cerebellar atrophy, hypotonia |
| COG4-CDG | Conserved oligomeric Golgi subunit 4 | COG4 | — | Mild mental retardation, mild dysmorphia, epilepsy, recurrent respiratory infections, mild ataxia |
| COG6-CDG | Conserved oligomeric Golgi subunit 6 | COG6 | — | Early fatality, severe neurological impairment, seizures, vomiting, intracranial bleeding, |
| ATP6V0A2-CDG | Golgi pH regulator | ATP6V0A2 | 219200 | Cutis laxa, wrinkly skin, connective tissue weakness, large fontanelle, variable mental retardation |
| Achondrogenesis type 1A | Golgi structure | GMAP210 | 200600 | Soft skull bones, short ribs that fracture easily, extremely short limbs, and lack normal ossification in the spine and pelvis |
| Gerodermia Osteodysplastica | Golgi structure | SCYL1BP1 | 231070 | Wrinkley lax skin, osteoporosis, variable growth retardation |
| Congenital dyserythropoietic anemia (CDA II) | ER-Golgi protein trafficking | SEC23B | 224100 | Anemia, jaundice, splenomegaly, gall bladder disease |
| Cranio-lenticulo-sutural dysplasia (CLSD) | ER-Golgi protein trafficking | SEC23A | 607812 | Facial dysmorphisms, sutural cataracts and skeletal defects |
| O-mannose disorders (dystroglycanopathy) | ||||
| Walker–Warburg syndrome | O-mannosyltransferase | POMT1, POMT2 | 268870 | Death in infancy, severe muscle weakness, diminished psychomotor development, abnormal neuronal migration ocular abnormalities |
| Muscle–eye–brain disease (MEB) | O-mannosyl GlcNAc transferase | POMGnT1 | 253280 | Severe muscle weakness, mental retardation, epilepsy, neuronal migration disorder, ocular abnormalities |
| Fukuyama-type congenital muscular dystrophy (FCMD) | Putative glycosyltransferase | Fukutin | 253800 | Severe proximal and axial weakness, mental retardation, epilepsy and abnormal neuronal migration |
| Congenital muscular dystrophy type 1C (MDC1C) | Fukutin-related protein, a putative glycosyltransferase | FKRP | 606612 | Hypotonia, impaired motor development with respiratory muscle weakness |
| Congenital muscular dystrophy type 1D (MDC1D) | Putative glycosyltransferase | LARGE | 608840 | Muscular dystrophy with severe mental retardation |
| Glycophosphatidyl inositol disorders | ||||
| Autosomal Recessive GPI Anchor Deficiency | 1st mannosyltransferase in GPI biosynthesis | PIGM | 610273 | Venous thrombosis and seizures |
| Hyperphosphatasia mental retardation (HPMR) syndrome | 2nd mannosyltransferase in GPI biosynthesis | PIGV | 239300 | Hyperphosphatasia, mental retardation, and distinct facial characteristics |
| Glycosphingolipid disorders | ||||
| Amish infantile epilepsy | Sia2,3Galβ1,4Glc-Cer synthase (GM3) | SIAT9 | 609056 | Neurological decline with tonic-clonic seizures and arrested development |
| Glycosaminoglycan synthesis disorders (O-xylose) | ||||
| Multiple hereditary exostoses | Heparan sulfate copolymerase | EXT1, EXT2 | 133701 | Bony outgrowths |
| Ehlers–Danlos syndrome progeroid form | Xylosylprotein β-1,4-galactosyltransferase | B4GALT7 | 604327 130070 | Connective tissue abnormalities with loose skin, hypotonia and developmental delay |
| Diastrophic dysplasia achondrogenesis | Anion (sulfate) transporter | DTDST | 600972 606718 | Premature calcification, scoliosis, club foot |
| Spondylo-epimetaphyseal dysplasia | 3′-phosphoadenosine- 5′-phosphosulphate synthase (PAPS) | ATPSK2 | 603005 | Abnormal skeletal development and linear growth |
| Spondylo-epimetaphyseal dysplasia (Omani type) | Chondroitin 6-O-sulfotransferase | CHST3 | 143095 608637 | Normal intelligence, reduced adult height, progressive kyphoscoliosis, joint dislocations, cardiac involvement, mild brachydactyly, camptodactyly, and microdontia |
| Macular corneal dystrophy | GlcNAc-6-sulfotransferase | CHST6 | 605294 | Progressive corneal opacity. |
| Adducted thumb-clubfoot syndrome | N-acetylgalactosamine 4-O-sulfotransferase 1 | CHST14 | 601776 | Congenital contractures of thumbs and feet with joint instability, facial clefting, coagulopathy, abnormal skin, heart, kidney, or intestinal defects |
| Schneckenbecken dysplasia | UDP-GlcA / UDP-GalNAc Golgi transporter | SLC35D1 | 610804 | Severely shortened long bones with bowing of limb bones and unossified vertebral bodies |
| O-GalNAc disorders | ||||
| Familial tumoral calcinosis | GalNAc transferase | GALNT3 | 211900 | Large calcium deposits in both skin and tissue |
| Tn syndrome | Chaperone of β1,3GalT | COSMC | 230430 | Hematological abnormalities including anemia, leucopenia, thrombocytopaenia |
| O-fucose disorders | ||||
| Peters plus syndrome | β-1,3 glucosyltransferase specific for O-fucose on thrombospondin type 1 repeats | B3GALTL | 261540 | Mental retardation with prenatal growth delay, short stature, brachymorphism, short limbs, and eye abnormalities |
aData adapted from Jaeken et al. 2009.
N-LINKED PATHWAY
Glycan trimming and reconstruction are unique to the N-glycosylation pathway as discussed in Stanley (2011). Mutations in the first α-glucosidase (glucosidase I) prevent the removal of the first glucose, but a backup cis- to medial Golgi endo-α-mannosidase is up-regulated (Volker et al. 2002) and cleaves an intact tetrasaccharide from the glycoproteins. This allows further processing to occur, and in contrast to many other N-glycosylation disorders, the typical serum glycoprotein structures such as those seen on transferrin are not altered. In fact, the disorder was only identified from the accumulation of the tetrasaccharide (Glcα1,2Glcα1, 3Glcα1,3Manα1,2) in the urine. The enzyme is transported from ER to Golgi in COP II vesicles. (Torossi et al. 2010).
Given the multiple glycosyltransferases involved in N-glycan processing and branching, this portion of the pathway might be expected to show many defects. Surprisingly, only two are known. One (CDG-IIa) results from essentially total loss of β-1,2-N-acetylglucosaminyltransferase (MGAT2) function. This enzyme is required for formation of multiantennary glycans and further processing into complex N-glycans. The few patients with this N-glycan-specific deficiency have severe psychomotor and growth retardation along with distinct abnormal facial features and thoracic deformity (Schachter and Jaeken 1999). The other defect (CDG-IId) is caused by mutation in β1,4 galactosyltransferase-I. The single patient had severe neurologic disease, myopathy, and clotting defects (Hansske et al. 2002). A series of other known β1,4 galactosyltransferases with different substrate preferences were insufficient to overcome this lesion, pointing to this enzyme as the major one required for N-glycan processing. This transferase is also responsible for extending GlcNAcβ1,3Fuc glycans necessary for fringe modulation of Notch signaling, mentioned below (Chen et al. 2001). This example underscores that mutations in a single transferase can affect multiple glycosylation pathways.
A special case of a Golgi transferase deficiency is I-cell disease or Mucolipidosis II, which can arguably be called the first N-glycosylation-specific disorder to be solved in 1981 (Reitman et al. 1981; Kollmann et al. 2010). It was categorized as a “lysosomal storage disorder” because cells accumulated inclusions of undegraded material, but in contrast to most such disorders with single enzyme deficiencies, the patients' cells lacked multiple lysosomal enzymes. Instead, patients' plasma or media from cultured cells accumulated multiple mislocalized, lysosomal enzymes suggesting a shared deficiency in localization. Although lysosomal enzymes share little sequence homology, they contain features that allow recognition by an oligomeric protein complex that transfers GlcNAc-1-P from UDP-GlcNAc to selected mannose units on high mannose type glycans on those enzymes (Kornfeld 1992). Subsequently a specific α-N-acetylglucosaminidase, an “uncovering enzyme,” cleaves that phosphodiester bond to generate one or two terminal mannose-6-P residues, which are ligands for Man-6-P receptors in the Golgi. This recognition marker allows for the delivery of the enzymes to the lysosome and the pH-dependent dissociation of the receptor and its recycling to the Golgi. Two receptors with different binding specificities can recognize the phosphorylated enzymes and with some preference for their different protein ligands. I-cell disease results from mutations in the GlcNAc-1-P phosphotransferase, not the “uncovering enzyme” (Kollmann et al. 2010).
This is the best example of protein-specific glycosylation that relies on structural features of the protein acceptor to allow high-affinity binding to modify glycan chain. Even simple mannose derivatives can serve as an acceptor for GlcNAc-1-P transferase reaction using this enzyme, but with 1000-fold lower efficiency (Lang et al. 1985).
TRANSPORTERS OF ACTIVATED DONORS
The activated nucleotide sugars made in the cytoplasm (or nucleus for CMP-sialic acid) must be transported into the Golgi lumen to glycosylate proteins and lipids. A series of membrane embedded, non-energy-dependent transporters are antiporters that import the nucleoside diphosphates and return the spent nucleoside monophosphates back to the cytoplasm (Liu et al. 2010). The transporters are present from yeast to mammalian cells, and are mostly localized to the Golgi. They can be highly substrate specific or show selectivity for a limited series of substrates for multiple glycosylation pathways (Hiraoka et al. 2007; Caffaro et al. 2008).
Congenital disorder of glycosylation-IIc is caused by mutations in the GDP-Fucose transporter. Patients have severe retardation and chronic infections. Some N- and O-glycans were hypofucosylated, but loss of a fucosylated glycan, sialyl LewisX, results in high levels of circulating neutrophils even in the absence of infections (Marquardt et al. 1999; Yakubenia et al. 2008). This glycan is required for leukocyte rolling prior to their extravasation into the tissues. For some patients, supplementing their diet with fucose corrected this deficiency. It is likely that another fucose transporter exists, but this has not been shown (Hellbusch et al. 2007). It is possible that another known transporter also uses GDP-fucose, but less efficiently (Liu et al. 2010).
A defect in the CMP-sialic acid transporter was reported in one patient who showed developmental delay and giant megakaryocytes (Martinez-Duncker et al. 2005).
Schneckenbecken dysplasia is caused by mutations in the dual substrate Golgi transporter for UDP-glucuronic acid and UDP-N-acetylgalactosamine that provides precursors for chondroitin sulfate synthesis (see below) (Hiraoka et al. 2007). Surprisingly, this protein appears to be localized in the ER. Loss-of-function mutations cause severe skeletal dysplasia with very short long bones (Hiraoka et al. 2007).
O-MANNOSE PATHWAY
For years, clinicians characterized mutations that cause Duchenne and Becker muscular dystrophies. They also identified a set of rare muscular dystrophies with variable clinical severity that often involved neurological abnormalities and eye defects, while other patients were neurologically normal (Jimenez-Mallebrera et al. 2005; Muntoni et al. 2008; Chandrasekharan and Martin 2010). This broad spectrum of disorders is caused by defects in a recently appreciated glycosylation pathway that is primarily focused on α-dystroglycan (αDG), a peripheral membrane component of the dystrophin–glycoprotein complex (DGC) located in muscle, nerve, heart, and brain. Relatively little work has been performed in studying the Golgi in muscular dystrophies, but see review by Percival and Froehner (2007).
The severe muscular dystrophies including muscle–eye–brain (MEB) disease, Fukuyama-type congenital muscular dystrophy (FCMD), Walker–Warburg syndrome, and limb–girdle muscular dystrophy, (Table 2). These mutations also appear to affect neuronal migration in the developing brain, thus accounting for the combined effects on muscle and brain development. The first sugar, Mannose-α-Ser/Thr, is added in the ER by POMT1/POMT2 complex and it is elongated in the Golgi by a β1,2 GlcNAc transferase (POMGNT1). Mutations in these genes decrease or eliminate enzyme activity. Mutations in fukutin (FKTN) and fukutin related protein (FKRP) also cause muscular dystrophy. These proteins have features and signatures of glycosyltransferases, but the specific reactions and acceptor substrates are unknown. Another protein called “Large” (because of its size) has two glycosyltransferase domains, but, again, the donor and acceptors are unknown (Barresi et al. 2004; Kanagawa et al. 2004). Besides the glycosyltransferase homology, a key tool in grouping these disorders is a set of monoclonal antibodies that recognize an undefined O-mannose-based glycan (Martin 2007). All of these disorders decrease the apparent molecular weight of αDG to the same extent and abolish antibody reactivity.
An important insight into the glycan structure and, therefore, genes and possible biosynthetic enzymes was that LARGE-dependent antibody-reactive glycans contain Man-6-P in a diester linkage to an unknown molecule. In the absence of LARGE-dependent modification of the glycan, Man-6-P exists as a monoester (Yoshida-Moriguchi et al. 2010). Why is this significant? Addition of Man-6-P to αDG does not use the lysosomal enzyme pathway that employs high-mannose type glycan acceptors. Here Mannose-O-Thr/Ser glycans are the acceptors. This glycan can be further extended with GalNAc or with Gal and Sia (Yoshida-Moriguchi et al. 2010). The latter can also be branched. O-mannose glycans account for one-third of all O-linked species in the brain (Yuen et al. 1997), but the majority is probably on other proteins because brain-specific ablation of αDG has little effect on the amount of these glycans. One study suggests that LARGE may act on the O-mannose, complex N-glycans, and mucin O-GalNAc glycans of αDG (Aguilan et al. 2009), so the story may be more complex.
O-XYL PATHWAY: GLYCOSAMINOGLYCANS
Xylβ-serine-linked glycans on selected proteins produce glycosaminoglycans (GAG) that share a common core tetrasaccharide GlcAβ1,3Galβ1,3Galβ1,4Xylβ. These linkage glycans are elongated into either chondrotin- and dermatan-sulfate composed of alternating (GalNAcβ1,4GlcAβ1,3) disaccharides or into heparan sulfate and heparin with alternating (GlcNAcα1,4GlcAβ1,4). Both polymers are variably sulfated and glucuronic acid (GlcA) units are epimerized to iduronic acid (IdU). The polymerization and modification occurs exclusively in the Golgi. Disorders in these pathways often cause skeletal- and chondrodysplasias.
Substrate Limitation
Sulfate ions must be imported into the cell because sulfate is not salvaged from degraded glycans. Several allelic clinical chondrodysplasias (diastrophic dysplasia, achondrogenesis type IB, neonatal osseous dysplasia I, and autosomal recessive multiple epiphyseal dysplasia), result from defective sulfation because of mutations in the sulfate ion transporter, encoded by DTDST (also known as SLC26A2) (Dawson and Markovich 2005). In spondyloepimetaphyseal dysplasia (SEMD), mutations occur in gene is ATPSK2, encoding the 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase (Sugahara and Schwartz 1979), the activated donor substrate for sulfation. A mouse model of this disease shows reduced limb length and axial skeletal size. In SEMD, there is progressive reduction in the size of the columnar and hypertrophic zones in the epiphyseal growth plates (ul Haque et al. 1998).
Disorders in Chain Initiation
Mutations in B4GALT7 cause the progeria variant of Ehlers–Danlos syndrome. The gene product (β1,4-Galactosyltransferase) (Okajima et al. 1999) adds the first Gal residue to xylose to the linkage region of GAG chains (Lindahl 1966) and only a few patients have been described (Quentin et al. 1990; Okajima et al. 1999; Faiyaz-Ul-Haque et al. 2004; Gotte and Kresse 2005). A dermatan sulfate proteoglycan from one patient's fibroblasts contained only xylose (Quentin et al. 1990).
Disorders in Chain Elongation—Hereditary Multiple Exostosis
The most common and well-studied GAG-related disorder is hereditary multiple exostosis (HME), caused by mutations in the synthetic machinery used for heparan sulfate (HS). It is one of the few autosomal dominant diseases and has a prevalence of about 1:50,000 (Schmale et al. 1994). More specifically, HME is caused by missense or frameshift mutations in either gene EXT1 or EXT2 that encode HS polymerases (Zak et al. 2002). HME patients develop bony outgrowths at the growth plates of the long bones. Normally the growth plates contain well-ordered chondrocytes in various stages of development, embedded in a collagen-chondroitin sulfate containing matrix (Zak et al. 2002). In HME patients, the outgrowths are composed of disorganized chondrocytes that must be surgically removed. These patients are at higher risk to develop osteosarcomas (Schmale et al. 1994) and some are diagnosed on the autistic spectrum (Li et al. 2002).
EXT1 and EXT2 appear to form a protein complex in the Golgi. Partial loss of one allele of either gene appears sufficient to cause MHE, which is unusual because most glycan biosynthetic enzymes are produced in excess.
Loss of HS synthesis, and the concurrent decrease in tissue HS, probably leads to an abnormal distribution of HS-binding growth factors. These include several members of the FGF family, and morphogens, such as hedgehog (Hh), decapentaplegic (Dpp), and wingless (Wg) (Esko and Selleck 2002). In Drosophila, loss of HS disrupts Hh, Wg, and Dpp pathways (Bornemann et al. 2004). Embryonic lethality and failure to gastrulate occur in mice that are null for either Ext gene (Lin et al. 2000; Stickens et al. 2005). However, Ext2 heterozygous animals are viable. These develop a single visible exostosis on the ribs in about a third of the litters (Stickens et al. 2005). Hh signaling in these animals is normal because no difference was detected in the protein distribution based on immunohistochemistry, thus not explaining the phenotype. Ext1 heterozygotes also develop exostoses, but the penetrance is strain-dependent, also seen within different families who carry the same mutations, suggesting a profound modifier gene effect.
Macular corneal dystrophy is caused by a deficiency in a tissue-specific sulfotransferase (CHST6), corneal N-acetylglucosamine-6-sulfotransferase (GlcNAc6ST), which is responsible for the production of corneal keratan sulphate (Akama et al. 2000). Unsulfated keratan chains are poorly soluble and their eventual precipitation disrupts the collagen network, leading to thinning and loss of transparency of the corneal stroma.
Mutations in another sulfotransferase gene (CHST14) are responsible for the addition of 4-O-sulfate to GalNAc residues in dermatan sulfate (DS) (Dundar et al. 2009). Its loss causes facial dysmorphism, adducted thumbs, clubbed feet, joint instability, short stature, and coagulopathy. Several patients died of respiratory insufficiency. Patient fibroblasts were missing highly flexible DS chains, but had an overabundance of less flexible chondroitin sulfate (CS), which likely affected collagen bundle formation or maintenance. The first biosynthetic step of converting CS to DS is the epimerization of glucuronic acid in CS to iduronic acid, and it is easily reversible. The sulfotransferase appears to “lock in” the commitment toward DS synthesis, but without it, reverse epimerization regenerates CS (Miyake et al. 2010). This discovery stresses the importance of dermatan sulfate in human development and matrix maintenance.
O-FUCOSE DISORDERS
Mutations in the β1,3-glucosyltransferase, B3GALTL, result in the autosomal recessive disorder Peters plus syndrome that is characterized by developmental delay, short stature, craniofacial defects and most frequently by anterior eye-chamber abnormalities (Lesnik Oberstein et al. 2006). When the genetic defect for Peters plus syndrome was identified, the precise function for B3GALTL was unknown. Based on protein sequence homology the protein was hypothesized to be a galactosyltransferase, but shown experimentally to be a glucosyltransferase (Kozma et al. 2006; Sato et al. 2006). Later, Hess et. al. determined that B3GALTL was involved in the addition of a glucose to O-linked fucose to form glucose-β1,3-fucose disaccharide associated specifically with thrombospondin type-1 repeats (Hess et al. 2008).
Thrombospondin type-1 repeats (TSRs) are a class of protein motifs similar to epidermal growth factor-like (EGF) repeats and are comprised of a cysteine rich domain ∼40–60 amino acids involved in several biological processes such as proper coagulation, cell migration, neurogenesis, and angiogenesis (Tucker 2004).
Although TSRs are structurally and functionally similar to EGF repeats, separate and distinct enzymes carry out the modification. Addition of fucose to an EGF repeats, as in the case of NOTCH, is performed solely by protein O-fucosyltransferase 1 (POFUT1). Whereas fucose modification of TSR is only performed by protein O-fucosyltransferase 2 (POFUT2) and further extended by B3GALTL to yield a unique disaccharide glucose-β1,3-fucose (Luo et al. 2006).
It is suggested that O-fucosylation of TSR-containing proteins regulates the proper folding of secreted proteins and dysfunction would lead to accumulation of misfolded proteins and diminished secretion (Ricketts et al. 2007). Analysis of several TSRs containing proteins from Peters plus patients revealed a complete lack of O-fucosylation, supporting Peters plus syndrome as a glycosylation disorder (Hess et al. 2008).
O-GALNAC DISORDERS
Familial tumoral calcinosis (FTC) is an autosomal recessive disorder characterized by painfully large calcium deposits, hyperphosphatemia, and debilitating secondary side affects such as recurrent infection. Genome wide marker analysis of two large unrelated families helped to identify the glycosyltransferase, GALNT3, as the causative gene mutated in FTC (Topaz et al. 2004).
GALNT3 belongs to a large family of UDP-GalNAc transferase localized to the cis-Golgi and catalyzes the transfer of N-acetylgalactosamine to a serine or threonine residue allowing for the initiation of mucin O-glycosylation. Although there are several genes with high sequence homology, it appears GALNT3 possesses a unique acceptor substrate specificity not seen in other family members which can clearly be seen in its ability to glycosylate both fibronectin and HIV gp120 (Matsuura et al. 1988; Bennett et al. 1999).
Mutations in the O-glycosylated protein, Fibroblast growth factor 23 (FGF23), result in another form of FTC. Two separate groups identified cases in which GALNT3 was normal, but potential O-glycosylated serine residues in FGF23 were mutated. They subsequently showed that mutagenesis of either serine residue dramatically impaired O-glycosylation and for the first time proved certain mutations in FGF23 resulted in FTC (Larsson et al. 2005; Bergwitz et al. 2009).
Another O-GalNAc deficiency is the rare autoimmune disease, Tn syndrome. It is caused by somatic mutations in the X-linked gene, COSMC and is characterized by various hematological abnormalities (Crew et al. 2008). COSMC is a chaperone for β1,3 galactosyltransferase and is necessary for the formation of the glyco-structure, T-antigen (Ju and Cummings 2002). T-antigen is an O-linked Galβ1,3GalNAc that acts as a precursor for most mucin O-glycans and can be further extended into several other structures. Mutations in COSMC result in the formation of a truncated structure known as Tn Antigen that is simply an O-linked linked GalNAc (Ju and Cummings 2005; Ju et al. 2008).
GLYCOSPHINGOLIPID DISORDERS
Autosomal recessive Amish infantile epilepsy is associated with developmental delay, seizures, and blindness. Linkage analysis of a large Amish family identified a nonsense mutation in SIAT9 resulting in a stop codon and consequently truncated protein (Simpson et al. 2004). SIAT9 is a sialyltransferase that makes ganglioside GM3 (Siaα2,3Galβ1,4Glc-ceramide) from lactosylceraminde (Galβ1,4Glc-ceramide). Hence, it is not surprising that patients with the truncated SIAT9 showed accumulation of nonsialylated plasma glycosphingolipids, and of GM3 precursors and lacked downstream GM3-dependent products. Interestingly, GM3 synthase null mice do not duplicate the human disorder because of an alternative biosynthetic pathway (Yamashita et al. 2003).
GLYCOSYLPHOSPHATIDYLINOSITOL ANCHOR DISORDERS
Inherited GPI deficiency (IGD) is characterized by venous thrombosis in addition to seizures and is caused by a mutation in the mannosytransferase PIGM that is required for the addition of the first mannose to the core glycosylphosphatidylinositol (GPI) (Maeda et al. 2001). In two unrelated consanguineous families, Almeida and colleagues identified a shared promoter mutation in a key SP1 transcription factor binding site that dramatically reduced the expression of PIGM leading to a defect in GPI anchor synthesis (Almeida et al. 2006).
In a follow-up paper involving the same patients, Almeida and colleagues showed patient lymphoblast treated with sodium butyrate completely restored normal PIGM transcript level as well as cell surface GPI expression. More importantly, treating the patient with sodium phenylbutyrate, a histone deacetylase inhibitor, resulted in a dramatic improvement after only two weeks, as measured by a lack of seizure activity and improved motor skills (Almeida et al. 2007).
Mutations in PIGV that encode the second mannosyltransferase of GPI anchors cause hyperphosphatasia mental retardation syndrome (a.k.a. Mabry syndrome, OMIM 239300, Rasmussen 1975; Krawitz et al. 2010). Patients with these mutations have greatly increased plasma alkaline phosphatase, a GPI-anchored protein, along with unusual facial features, variable seizures, and muscular hypotonia (Horn et al. 2010). Surface expression of GPI-anchors themselves and anchored proteins were reduced in patients' leukocytes. PIGV-deficient CHO cells can be complemented with wild-type human allele, but not with the mutant.
Somatic mutations in PIGA limit the first step of GPI synthesis resulting in partial or complete loss of GPI-linked membrane proteins and are the underlying cause of Paroxysmal nocturnal hemoglobinuria (PNH), an acquired hematopoietic disorder characterized by unusual blood cell populations and intravascular hemolysis (Takeda et al. 1993).
FACTORY ORGANIZATION, SHIPPING, AND RECEIVING
It is not surprising that mutations in the glycosylation hardware disrupt patients' glycosylation pathways. Some enzymes in glycosylation pathways form complexes in the Golgi (Opat et al. 2000; Pinhal et al. 2001; Sprong et al. 2003; Young 2004; Maccioni 2007; Hassinen et al. 2010), which presumably increases efficiency of substrate modification. However, mutations in genes that disrupt Golgi organization, structure, and trafficking of either cargo or resident proteins also cause disorders that affect glycosylation. Perhaps this should be expected because drugs that alter Golgi integrity and protein trafficking also alter glycosylation. Therefore, altered glycosylation may be a surrogate indicator of the functional importance of a Golgi-related protein. Many disease-causing mutations in specific proteins (Howell et al. 2006) cause protein misfolding, retention in the ER, and proteasomal degradation. Examples include, α1-antitrypsin deficiency, autosomal dominant polycystic kidney disease, cystic fibrosis, Charcot-Marie-Tooth disease, congenital sucrase-isomaltase deficiency, Fabry's disease, familial hypercholesterolemia, and osterogenesis imperfecta (Vogt et al. 2005; Howell et al. 2006). An unusually high proportion (1.4%) of such mutations in proteins traveling the ER/Golgi circuit actually create inappropriate N-glycosylation sites. Some of these are pathological. Their delivery to the membrane is normal (Vogt et al. 2005; Vogt et al. 2007), but the presence of the glycan actually impairs function. Alternatively, the mutations may prevent the assembly into multisubunit complexes such as in the case of fibrillin1 in Marfan's syndrome (Raghunath et al. 1995; Lonnqvist et al. 1996).
It is more challenging to find the appropriate cell/tissue or specific protein that shows the alteration and identifies a mechanism. Serum proteins (hepatocyte and plasma cell-derived) are convenient markers, but they may not always indicate defective glycosylation. Impairments may only appear in selected tissues where the “glycosylation demand” is high.
COGs AND GLYCOSYLATION
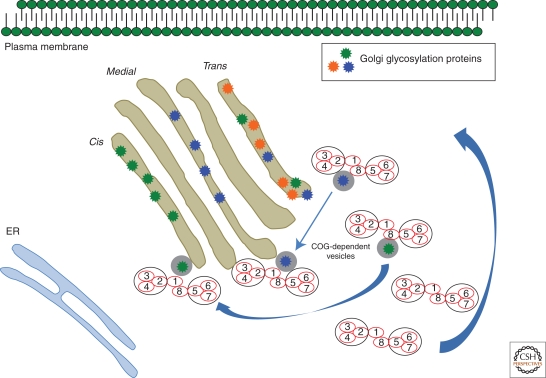
Conserved oligomeric Golgi (COG) complex is composed of eight individual subunits, COG1–8. Mutations are known for six subunits, making it the best known of the trafficking defects (Ungar 2009). The complex exists in two lobes or subcomplexes. Lobe A (COG1–4) and Lobe B (COG 5–8) are bridged by a COG1/COG8 heterodimer that links the different lobes. COG1 and COG2 were identified as the defective genes in ldlB and ldlC mutant CHO lines, respectively, that were defective in the surface localization of the LDL receptor. These lines had incomplete N- and O-linked glycans and abnormal Golgi architecture. The COG complex localizes to the cis- and medial Golgi and is found at the tips and along the rims of the cisternae and on vesicles, making it a likely tethering factor (Vasile et al. 2006). See Figure 1.
Figure 1.
Role of the conserved oligomeric Golgi (COG) complex in Golgi localized retrograde transport. In focus is how the COG complex transports various mislocalized Golgi glycosylation proteins back to their proper destinations and then returns after redirecting the vesicles.
The COG complex is found from yeast to mammals and interacts with other trafficking proteins. For instance, COG3 coprecipitates with β-COPI and in yeast, COG2 interacts with γCOPI. When COG3 is depleted using siRNA knockdown, vesicles containing glycosylation enzymes, GPP130 (cis-Golgi) and the SNAREs GS15 and GS28 accumulate. COGs appear to be important for retrograde trafficking because COG depletion cannot move cell surface bound bacterial toxins (e.g., Shiga and AB) through the Golgi to the ER (Smith et al. 2009).
SM (SEC1/MUNC18) protein, Sly1, interacts directly with (COG4) which also interacts with Syntaxin5 using different binding sites. Blocking COG4-Sly1 interaction impairs pairing of SNAREs involved in intra-Golgi transport thereby markedly attenuating Golgi-to-ER retrograde transport (Laufman et al. 2009). The interaction between p115 and COG2 is essential for Golgi ribbon reformation after the disruption of the ribbon by p115 siRNA knockdown or brefeldin A treatment (Sohda et al. 2007).
In a broad screen of Rab binding factors mammalian COG6 bound to GTP-restricted Rab1, Rab6, and Rab41 and COG4 preferentially interacts with Rab30-GTP (Fukuda et al. 2008).
The first sib patients with a COG-based trafficking disorder showed defective N- and O-glycosylation caused by splice mutations in COG7 (Wu et al. 2004). The patients had perinatal asphyxia, feeding problems, growth retardation, hypotonia, enlarged cholestatic liver, epileptic seizures, cerebral atrophy, and severely shortened limbs with absence of long bone epipheses. Another curious feature was an accumulation of excessive, wrinkled, doughy-like skin. Both patients died of infections and cardiac insufficiency. Six additional COG7-deficient patients with this mutation showed similar severe phenotypes (Morava et al. 2007; Ng et al. 2007). One patient with a different splice site mutation had no dysmorphic features and prolonged survival (Zeevaert et al. 2009a). A COG1-deficient patient was identified with generalized hypotonia, but with normal strength, failure to thrive, small hands and feet, and short stature (Foulquier et al. 2006) Cerebellar and cerebral abnormalities were milder than in COG7 patients. The mutation eliminated the carboxy-terminal 80 amino acids. Two other COG1-deficient patients were found with costovertebral dysplasia in cerebrocostomandibular syndrome (CCMS) and in cerebrofaciothoracic dysplasia and spondylocostal dysostosis (Zeevaert et al. 2009b).
Two patients were identified with COG8 mutations. One with a truncating mutation that eliminated 76-carboxy-terminal amino acids had a normal neonatal and infancy, but later developed mental retardation and ataxia from cerebellar atrophy/hypoplasia (Foulquier et al. 2007). The other patient had a splice site mutation and a deletion that truncated the protein (Kranz et al. 2007). This patient is severely retarded and has a near absence of muscle that resembles malnutrition, but no dysmorphic features. COG5-deficient patient had a splicing mutation that caused exon skipping and severe reduced expression of the COG5 protein, but with mild psychomotor retardation, delayed motor and language development (Paesold-Burda et al. 2009).
COG4 deficiency (Reynders et al. 2009; Richardson et al. 2009) in one patient with a missense mutation and small deletion caused only mild phenotype. Its loss decreased the expression or stability of the other Lobe A subunits, but stability of the complex was still seen in the cytoplasm at lower levels. Another patient with a COG4 defect had a fatal outcome (Ng et al. 2011). A patient has also been identified with lethal mutations in COG6 that caused neurologic disease, seizures, and intracranial bleeding (Lubbehusen et al. 2010).
Fibroblasts from all COG patients show a three to fourfold slower rate of brefeldin A–induced Golgi dissociation, while postwashout Golgi reassembly is normal. This is consistent with COG's role in retrograde transport from Golgi to ER. In all cases except COG4, expression of the normal allele in patients' cells normalizes BFA response (Foulquier 2009; Reynders et al. 2009).
The very broad spectrum of clinical presentations among COG patients may seem puzzling, and although it may be lumped into the “genetic background” explanation, other issues may cloud predictions or interpretations. As with most glycoyslation disorders, the mutated alleles are hypomorphic, not null. Also, patient fibroblasts are convenient, but probably not reflective of the pathology in other cells or tissues. This is especially important because COG mutations frequently involve splice sites that can show variable penetrance. Finally, understanding of COG cell biology is in an early stage, and it is clear that COGs function in intra-Golgi as well as Golgi to ER transport, but it is not known if all the components are required for each role.
Little is known about how the COG complex maintains proper glycosylation, homeostasis, and structural integrity of the Golgi. Recent analysis suggests that the amino-terminal portion of Cogs1–4 are required for assembly of a core complex, whereas the carboxy-terminal domains form “elongated legs” that interact with other glycosylation related proteins. Carboxy-terminal domain of COG1 is required for interaction with the COG 5–8 subunit complex (Lees et al. 2010). Another study shows that small interfering RNA knockdown of lobe A (Cogs1–4) subunits alter Golgi structure, whereas loss of Lobe B (Cogs 5–8) subunits decreased stability of both B4GALT1 and ST6GAL1 (Peanne et al. 2010) used for serum glycoprotein synthesis. COG patients show deficiencies in both galactosylation and sialylation.
Other genetic disorders will likely arise from defects in proteins that directly interact with the COG complex.
VACUOLAR ATPase
Autosomal recessive cutis laxa (ARCL) is associated with a progeroid appearance, lax and wrinkled skin, osteopenia, and mental retardation (Morava et al. 2008). One of the major diagnostic criteria is abnormal elastin fibers (Morava et al. 2009), but the genetic deficiencies of many cutis laxa patients were unknown. Mutations in ATP6V0A2, the a2 subunit of the vacuolar H+-ATPase (V-ATPase) were shown to affect N- and O-glycosylation and alter Golgi trafficking as determined by BFA treatment (Guillard et al. 2009; Kornak et al. 2008). Cells from patients had distended Golgi cisternae with accumulation of abnormal lysosomes and multivesicular bodies. Tropoelastin (TE) accumulated in the Golgi and in large, abnormal intracellular and extracellular aggregates. siRNA knockdown of ATP6V0A2 showed similar phenotype. The delayed secretion and increased intracellular retention of TE in the Golgi and reduced extracellular deposition of mature elastin increased apoptosis of elastogenic cells (Hucthagowder et al. 2009). It is likely that the vacuolar ATPase maintains correct pH in lysosomes, synaptic vesicles, endosomes, secretory granules, and the Golgi apparatus (Saroussi and Nelson 2009). Another candidate disease gene could be the Golgi pH regulator (GPHR) that shows a voltage-dependent anion-channel activity and alters glycosylation (Maeda et al. 2008).
GOLGINS
Golgins bind to the cytoplasmic side of cis- and trans-Golgi and can act as tethers using their coiled coil domains that project from the cisternae as long thin fibers (Ramirez and Lowe 2009; Sztul and Lupashin 2009; Goud and Gleeson 2010). They interact with small GTPases, and some bind to the cytoskeletal elements. Golgin GMAP210 recruits γ tubulin complexes to form Golgi ribbons and also interacts with Arf1 (Drin et al. 2008; Cardenas et al. 2009). GMAP210 binds to highly curved membranes and is thought to tether tubules or vesicles to the cis-Golgi (Drin et al. 2008). Overepression induces Golgi fragmentation and disturbs ER to Golgi trafficking (Ramirez and Lowe 2009). It is widely expressed and may be redundant because deficient mouse embryos have a normal Golgi. This protein anchors the cilia component of IFT20 to the Golgi and these cells from the mutants have shorter primary cilia, suggesting that it plays some role in the sorting of membrane components to the cilia (Follit et al. 2008). This conclusion matched the known data, but did not explain why GMAP210-deficient mice died soon after birth, apparently of heart and complex lung defects (Follit et al. 2009). The answer emerged from a forward genetic screen focused on determining the genes responsible for neonatal lethal skeletal defects in mice (Smits et al. 2010). The target gene Trip11 encoded GMAP210 and the mouse phenotype resembled that of a human disorder achondrogenesis type Ia (Smits et al. 2010). Subsequent analysis of patients showed mutations in TRIP11. The phenotype could not have been predicted from the cell biology, especially because GMAP210 is widely expressed. Chondrocytes from mutant mice show impaired differentiation, distended ER, and altered glycosylation. Many of the large matrix proteins such as type II collagen and aggrecan were correctly localized, but perlecan, a large heparan sulfate proteoglycan accumulated in the ER. This argued that GMAP210 deficiency caused a selective protein trafficking defect in a tissue that carried a heavy demand for glycosylation of proteoglycans. Early death seemed to result from lung compression caused by an underdeveloped skeleton rather than a defect in the primary cilium assembly and localization. There is evidence that golgins can play a specific role in cargo protein selection (Drin et al. 2008; Ungar 2009). This example is important because it points out the difficulty of predicting which proteins, organ systems, or time frames will be affected by mutations in Golgi trafficking proteins.
Gerodermia osteodysplastica is a genetic disorder that causes premature wrinkly skin and osteoporosis, defining it as a progeroid disorder (Al-Gazali et al. 2001). Mapping studies localized the defect to SCYL1BP1 loss-of-function mutations. The protein is highly expressed in those locations and it increases during osteoblast differentiation (Hennies et al. 2008). Immunofluorescence localized it to the Golgi, and yeast two-hybrid pull down showed it interacted with RAB6, in its GTP-activated state. The protein contained coiled coil domains, localized to the Golgi, and bound RAB6, identifying it as a golgin. These results reinforce the relation of the secretory pathway in selected tissues with age-related changes in connective tissues (Hennies et al. 2008). Additional patients had similar phenotypes and mutations in this gene, but transferrin glycosylation was normal (Al-Dosari and Alkuraya 2009). As seen with GMAP210 mutations that cause achondrogenesis type Ia, the effects on glycosylation may be protein selective and tissue specific.
SEC PROTEINS
Another example showing unpredictable functional consequences is also seen in two disorders caused by mutations in the family of COPII coat protein complexes, SEC23A and SEC23B (Lang et al. 2006; Bianchi et al. 2009). Mutations in SEC23A cause the cranio-lenticulo-sutural dysplasia syndrome and affect facial development (Boyadjiev et al. 2006). In zebrafish, positional cloning mapped the defect of a malformed craniofacial skeleton, kinked pectoral fins and a short body length to the homolog of sec23b. The mutant fish also showed accumulation of ECM components including type II collagen in the ER (Lang et al. 2006). Mice mutated in the transcription factor BBF2H7 that activates Sec23A transcription have a similar phenotype to GMAP210-deficient mice including abnormal chondrogenesis and accumulation of collagen II and cartilage oligomeric matrix protein in the ER. Introduction of Sec23A into chondrocytes normalized the secretion of matrix components (Stagg et al. 2008; Saito et al. 2009).
The mutant form of SEC23A poorly recruits the SEC13-SEC31 complex, inhibiting vesicle formation. Surprisingly, this effect is modulated by the SAR1 GTPase paralog used in the reaction, indicating distinct affinities of the two human SAR1 paralogs for the SEC13–SEC31 complex. Patient cells accumulate numerous tubular cargo-containing ER exit sites devoid of observable membrane coat, likely representing an intermediate step in COPII vesicle formation (Fromme et al. 2007).
In contrast, SEC23B mutations cause a completely different glycosylation disorder called congenital dyserythropoietic anemia or HEMPAS (Schwarz et al. 2009). These patients have poor erythropoiesis generating bi- and multinucleated erythroblasts in bone marrow, suggesting abnormal cytokinesis (Denecke and Marquardt 2009). In peripheral red blood cells, proteins and glycolipids are incompletely glycosylated (Fukuda 1990). This leads to a progressive splenomegaly, gallstones, and iron overload potentially with liver cirrhosis or cardiac failure. Knockdown of SEC23B via shRNA mimics the defective cytokinesis, and zebrafish morphants have abnormal enrythrocyte development (Bianchi et al. 2009; Schwarz et al. 2009). Surprisingly analysis of one family showed that heterozygous parents had detectable abnormalities in erythrocyte membrane glycans, but a healthy child was completely normal, thus suggesting the presence of a subclinical haploinsufficiency (Zdebska et al. 2002).
LIPID HOMEOSTASIS AND TRAFFICKING
Lipid and glycolipid trafficking also involve the Golgi together with other compartments in the cell (Lev 2006; Chandran and Machamer 2008; van Meer et al. 2008). Recent proposed explanations for the organization, dynamics, and flux of both proteins and lipids through the Golgi are based primarily on their physical properties, and sites of synthesis/depletion to construct membranes of different compositions, dimensions, and curvatures (Lippincott-Schwartz and Phair 2010). Lipid and protein sorting are interwoven and interdependent in this attractive, but unproven model (Emr et al. 2009; Gong et al. 2010). However, it provides a setting to discuss the possibility that altered glycosylation may be a useful marker or play a role in pathophysiology of the disorders. These include various disorders of cholesterol trafficking such as Niemann–Pick type C (Urano et al. 2008; Lloyd-Evans and Platt 2010). Defects in this protein of still unknown function also affect the esterification of dolichol (Turunen and Schedin-Weiss 2007) and glycosylation of ApoE in mouse models (Chua et al. 2010). Surprisingly, study of lipid storage and trafficking models in Drosophila, shows that cholesterol storage alters the trafficking of a glycolipid from the biosynthetic to the degradative endolysosomal pathway and cholesterol depletion eliminates glycolipid recycling. Lactosyl ceramide diverts a neutral cargo (dextran) away from the lysosome (Hortsch et al. 2010).
PERSPECTIVES AND CONCLUSIONS
Current and emerging gene sequencing technology as well as falling costs (Ng et al. 2009; Horn et al. 2010; Roach et al. 2010) will reveal new inherited disorders in the near future. The examples in this article show that Golgi-associated disorders that affect glycosylation will be well represented. Protein homology predictions may not produce accurate predictions of patients' phenotypes (e.g., compare SEC23A and SEC23B or PIGM vs. PIGV). Forward genetic screens in model organisms (GMAP210 causing lethal achondrogensis) may help identify interacting pathways and protein binding partners. Some of the defective genes will be familiar, others will have unknown functions or be poorly annotated making their physiological function difficult to unravel. In some cases, biochemical confirmation of altered glycosylation can be a quality control indicator that implicates the Golgi in the physiological functions of defective genes (Nilsson et al. 2009). Genetic defects commonly associated with various medical specialties will require increased interaction and cooperation with basic cell biologists and biochemists to explain and translate these genetic errors into physiology and potential treatments.
SUMMARY
The Golgi apparatus sorts, organizes, and traffics intracellular protein and lipid cargos. It glycosylates cargo and maintains factory organization for efficient pickups and deliveries between the ER and final destinations (plasma membrane or endocytic organelles). Impaired performance by mutated Golgi resident proteins creates severe and highly variable pathologies. Emerging developments in patients' genetic analysis should identify new Golgi components/processes, in which glycosylation is a good indicator of Golgi and the patients' health or pathology.
ACKNOWLEDGMENTS
The authors are supported by grant no. R01DK55615, The Rocket Williams Fund, and the Sanford Children's Health Research Center. H.H.F. is a Sanford Professor.
Footnotes
Editors: Graham Warren and James Rothman
Additional Perspectives on The Golgi available at www.cshperspectives.org
REFERENCES
- Aguilan JT, Sundaram S, Nieves E, Stanley P 2009. Mutational and functional analysis of Large in a novel CHO glycosylation mutant. Glycobiology 19: 971–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama TO, Nishida K, Nakayama J, Watanabe H, Ozaki K, Nakamura T, Dota A, Kawasaki S, Inoue Y, Maeda N, et al. 2000. Macular corneal dystrophy type I and type II are caused by distinct mutations in a new sulphotransferase gene. Nat Genet 26: 237–241 [DOI] [PubMed] [Google Scholar]
- Al-Dosari M, Alkuraya FS 2009. A novel missense mutation in SCYL1BP1 produces geroderma osteodysplastica phenotype indistinguishable from that caused by nullimorphic mutations. Am J Med Genet A 149A: 2093–2098 [DOI] [PubMed] [Google Scholar]
- Al-Gazali LI, Sztriha L, Skaff F, Haas D 2001. Gerodermia osteodysplastica and wrinkly skin syndrome: Are they the same? Am J Med Genet 101: 213–220 [DOI] [PubMed] [Google Scholar]
- Almeida AM, Murakami Y, Layton DM, Hillmen P, Sellick GS, Maeda Y, Richards S, Patterson S, Kotsianidis I, Mollica L, et al. 2006. Hypomorphic promoter mutation in PIGM causes inherited glycosylphosphatidylinositol deficiency. Nat Med 12: 846–851 [DOI] [PubMed] [Google Scholar]
- Almeida AM, Murakami Y, Baker A, Maeda Y, Roberts IA, Kinoshita T, Layton DM, Karadimitris A 2007. Targeted therapy for inherited GPI deficiency. N Engl J Med 356: 1641–1647 [DOI] [PubMed] [Google Scholar]
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang W, Schachter H, Dumanski JP, et al. 2004. LARGE can functionally bypass α-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med 10: 696–703 [DOI] [PubMed] [Google Scholar]
- Bennett EP, Hassan H, Mandel U, Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, van Kessel AG, Olofsson S, Clausen H 1999. Cloning and characterization of a close homologue of human UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem 274: 25362–25370 [DOI] [PubMed] [Google Scholar]
- Bergwitz C, Banerjee S, Abu-Zahra H, Kaji H, Miyauchi A, Sugimoto T, Juppner H 2009. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab 94: 4267–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P, Fermo E, Vercellati C, Boschetti C, Barcellini W, Iurlo A, Marcello AP, Righetti PG, Zanella A 2009. Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum Mutat 30: 1292–1298 [DOI] [PubMed] [Google Scholar]
- Bonnon C, Wendeler MW, Paccaud JP, Hauri HP 2010. Selective export of human GPI-anchored proteins from the endoplasmic reticulum. J Cell Sci 123: 1705–1715 [DOI] [PubMed] [Google Scholar]
- Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R 2004. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development 131: 1927–1938 [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, Hur DJ, Zhang G, Hamamoto S, Schekman R, Ravazzola M, et al. 2006. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet 38: 1192–1197 [DOI] [PubMed] [Google Scholar]
- Caffaro CE, Luhn K, Bakker H, Vestweber D, Samuelson J, Berninsone P, Hirschberg CB 2008. A single Caenorhabditis elegans Golgi apparatus-type transporter of UDP-glucose, UDP-galactose, UDP-N-acetylglucosamine, and UDP-N-acetylgalactosamine. Biochemistry 47: 4337–4344 [DOI] [PubMed] [Google Scholar]
- Cardenas J, Rivero S, Goud B, Bornens M, Rios RM 2009. Golgi localisation of GMAP210 requires two distinct cis-membrane binding mechanisms. BMC Biol 7: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran S, Machamer CE 2008. Acute perturbations in Golgi organization impact de novo sphingomyelin synthesis. Traffic 9: 1894–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan K, Martin PT 2010. Genetic defects in muscular dystrophy. Methods Enzymol 479: 291–322 [DOI] [PubMed] [Google Scholar]
- Chen J, Moloney DJ, Stanley P 2001. Fringe modulation of Jagged1-induced Notch signaling requires the action of β4galactosyltransferase-1. Proc Natl Acad Sci 98: 13716–13721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua CC, Lim ML, Wong BS 2010. Altered apolipoprotein E glycosylation is associated with Aβ(42) accumulation in an animal model of Niemann-Pick Type C disease. J Neurochem 112: 1619–1626 [DOI] [PubMed] [Google Scholar]
- Clement E, Mercuri E, Godfrey C, Smith J, Robb S, Kinali M, Straub V, Bushby K, Manzur A, Talim B, et al. 2008. Brain involvement in muscular dystrophies with defective dystroglycan glycosylation. Ann Neurol 64: 573–582 [DOI] [PubMed] [Google Scholar]
- Coman D, Irving M, Kannu P, Jaeken J, Savarirayan R 2008. The skeletal manifestations of the congenital disorders of glycosylation. Clin Genet 73: 507–515 [DOI] [PubMed] [Google Scholar]
- Crew VK, Singleton BK, Green C, Parsons SF, Daniels G, Anstee DJ 2008. New mutations in C1GALT1C1 in individuals with Tn positive phenotype. Br J Haematol 142: 657–667 [DOI] [PubMed] [Google Scholar]
- Dawson PA, Markovich D 2005. Pathogenetics of the human SLC26 transporters. Curr Med Chem 12: 385–396 [DOI] [PubMed] [Google Scholar]
- Denecke J, Marquardt T 2009. Congenital dyserythropoietic anemia type II (CDAII/HEMPAS): Where are we now? Biochim Biophys Acta 1792: 915–920 [DOI] [PubMed] [Google Scholar]
- Donaldson JG, McPherson PS 2009. Membrane trafficking heats up in Pavia. Golgi meeting on membrane trafficking in global cellular responses. EMBO Rep 10: 132–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Morello V, Casella JF, Gounon P, Antonny B 2008. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science 320: 670–673 [DOI] [PubMed] [Google Scholar]
- Dundar M, Muller T, Zhang Q, Pan J, Steinmann B, Vodopiutz J, Gruber R, Sonoda T, Krabichler B, Utermann G, et al. 2009. Loss of dermatan-4-sulfotransferase 1 function results in adducted thumb-clubfoot syndrome. Am J Hum Genet 85: 873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund EA, Freeze HH 2006. The congenital disorders of glycosylation: A multifaceted group of syndromes. NeuroRX 3: 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S, Glick BS, Linstedt AD, Lippincott-Schwartz J, Luini A, Malhotra V, Marsh BJ, Nakano A, Pfeffer SR, Rabouille C, et al. 2009. Journeys through the Golgi—Taking stock in a new era. J Cell Biol 187: 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko JD, Selleck SB 2002. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71: 435–471 [DOI] [PubMed] [Google Scholar]
- Faiyaz-Ul-Haque M, Zaidi SH, Al-Ali M, Al-Mureikhi MS, Kennedy S, Al-Thani G, Tsui LC, Teebi AS 2004. A novel missense mutation in the galactosyltransferase-I (B4GALT7) gene in a family exhibiting facioskeletal anomalies and Ehlers–Danlos syndrome resembling the progeroid type. Am J Med Genet A 128A: 39–45 [DOI] [PubMed] [Google Scholar]
- Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ 2008. The golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet 4: e1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Xu F, Keady BT, Pazour GJ 2009. Characterization of mouse IFT complex B. Cell Motil Cytoskeleton 66: 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier F 2009. COG defects, birth and rise! Biochim Biophys Acta 1792: 896–902 [DOI] [PubMed] [Google Scholar]
- Foulquier F, Vasile E, Schollen E, Callewaert N, Raemaekers T, Quelhas D, Jaeken J, Mills P, Winchester B, Krieger M, et al. 2006. Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. Proc Natl Acad Sci 103: 3764–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier F, Ungar D, Reynders E, Zeevaert R, Mills P, Garcia-Silva MT, Briones P, Winchester B, Morelle W, Krieger M, et al. 2007. A new inborn error of glycosylation due to a Cog8 deficiency reveals a critical role for the Cog1-Cog8 interaction in COG complex formation. Hum Mol Genet 16: 717–730 [DOI] [PubMed] [Google Scholar]
- Freeze HH 2006. Genetic defects in the human glycome. Nat Rev Genet 7: 537–551 [DOI] [PubMed] [Google Scholar]
- Freeze H, Elbein A 2009. Glycosylation precursors. In Essentials of glycobiology, 2nd ed. (ed. Varki A, et al. ), pp. 47–62 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Freeze H, Schachter H 2009. Genetic disorders of glycosylation. In Essentials of glycobiology, 2nd ed. (ed. Varki A, et al. ), pp. 585–600 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L 2007. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell 13: 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Maeda Y, Ra M, Yamaguchi Y, Taguchi R, Kinoshita T 2009. GPI glycan remodeling by PGAP5 regulates transport of GPI-anchored proteins from the ER to the Golgi. Cell 139: 352–365 [DOI] [PubMed] [Google Scholar]
- Fukuda MN 1990. HEMPAS disease: Genetic defect of glycosylation. Glycobiology 1: 9–15 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Ishibashi K, Itoh T 2008. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics 7: 1031–1042 [DOI] [PubMed] [Google Scholar]
- Gong H, Guo Y, Linstedt A, Schwartz R 2010. Discrete, continuous, and stochastic models of protein sorting in the Golgi apparatus. Phys Rev E Stat Nonlin Soft Matter Phys 81: 011914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M, Kresse H 2005. Defective glycosaminoglycan substitution of decorin in a patient with progeroid syndrome is a direct consequence of two point mutations in the galactosyltransferase I (β4galT-7) gene. Biochem Genet 43: 65–77 [DOI] [PubMed] [Google Scholar]
- Goud B, Gleeson PA 2010. TGN golgins, Rabs and cytoskeleton: Regulating the Golgi trafficking highways. Trends Cell Biol 20: 329–336 [DOI] [PubMed] [Google Scholar]
- Guillard M, Dimopoulou A, Fischer B, Morava E, Lefeber DJ, Kornak U, Wevers RA 2009. Vacuolar H+-ATPase meets glycosylation in patients with cutis laxa. Biochim Biophys Acta 1792: 903–914 [DOI] [PubMed] [Google Scholar]
- Haeuptle MA, Hennet T 2009. Congenital disorders of glycosylation: An update on defects affecting the biosynthesis of dolichol-linked oligosaccharides. Hum Mutat 30: 1628–1641 [DOI] [PubMed] [Google Scholar]
- Hansske B, Thiel C, Lubke T, Hasilik M, Honing S, Peters V, Heidemann PH, Hoffmann GF, Berger EG, von Figura K, et al. 2002. Deficiency of UDP-galactose:N-acetylglucosamine β-1,4-galactosyltransferase I causes the congenital disorder of glycosylation type IId. J Clin Invest 109: 725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen A, Rivinoja A, Kauppila A, Kellokumpu S 2010. Golgi N-glycosyltransferases form both homo- and heterodimeric enzyme complexes in live cells. J Biol Chem 285: 17771–17777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellbusch CC, Sperandio M, Frommhold D, Yakubenia S, Wild MK, Popovici D, Vestweber D, Grone HJ, von Figura K, Lubke T, et al. 2007. Golgi GDP-fucose transporter-deficient mice mimic congenital disorder of glycosylation IIc/leukocyte adhesion deficiency II. J Biol Chem 282: 10762–10772 [DOI] [PubMed] [Google Scholar]
- Hennies HC, Kornak U, Zhang H, Egerer J, Zhang X, Seifert W, Kuhnisch J, Budde B, Natebus M, Brancati F, et al. 2008. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet 40: 1410–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D, Keusch JJ, Oberstein SA, Hennekam RC, Hofsteenge J 2008. Peters plus syndrome is a new congenital disorder of glycosylation and involves defective O-glycosylation of thrombospondin type 1 repeats. J Biol Chem 283: 7354–7360 [DOI] [PubMed] [Google Scholar]
- Hiraoka S, Furuichi T, Nishimura G, Shibata S, Yanagishita M, Rimoin DL, Superti-Furga A, Nikkels PG, Ogawa M, Katsuyama K, et al. 2007. Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat Med 13: 1363–1367 [DOI] [PubMed] [Google Scholar]
- Horn D, Schottmann G, Meinecke P 2010. Hyperphosphatasia with mental retardation, brachytelephalangy, and a distinct facial gestalt: Delineation of a recognizable syndrome. Eur J Med Genet 53: 85–88 [DOI] [PubMed] [Google Scholar]
- Hortsch R, Lee E, Erathodiyil N, Hebbar S, Steinert S, Lee JY, Chua DS, Kraut R 2010. Glycolipid trafficking in Drosophila undergoes pathway switching in response to aberrant cholesterol levels. Mol Biol Cell 21: 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GJ, Holloway ZG, Cobbold C, Monaco AP, Ponnambalam S 2006. Cell biology of membrane trafficking in human disease. Int Rev Cytol 252: 1–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucthagowder V, Morava E, Kornak U, Lefeber DJ, Fischer B, Dimopoulou A, Aldinger A, Choi J, Davis EC, Abuelo DN, et al. 2009. Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Hum Mol Genet 18: 2149–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J, Matthijs G 2007. Congenital disorders of glycosylation: A rapidly expanding disease family. Annu Rev Genomics Hum Genet 8: 261–278 [DOI] [PubMed] [Google Scholar]
- Jaeken J, Hennet T, Matthijs G, Freeze HH 2009. CDG nomenclature: Time for a change! Biochim Biophys Acta 1792: 825–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mallebrera C, Brown SC, Sewry CA, Muntoni F 2005. Congenital muscular dystrophy: Molecular and cellular aspects. Cell Mol Life Sci 62: 809–823 [DOI] [PubMed] [Google Scholar]
- Ju T, Cummings RD 2002. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc Natl Acad Sci 99: 16613–16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Cummings RD 2005. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature 437: 1252. [DOI] [PubMed] [Google Scholar]
- Ju T, Aryal RP, Stowell CJ, Cummings RD 2008. Regulation of protein O-glycosylation by the endoplasmic reticulum–localized molecular chaperone Cosmc. J Cell Biol 182: 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB, et al. 2004. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell 117: 953–964 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Fujita M, Maeda Y 2008. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: Recent progress. J Biochem 144: 287–294 [DOI] [PubMed] [Google Scholar]
- Kollmann K, Pohl S, Marschner K, Encarnacao M, Sakwa I, Tiede S, Poorthuis BJ, Lubke T, Muller-Loennies S, Storch S, et al. 2010. Mannose phosphorylation in health and disease. Eur J Cell Biol 89: 117–123 [DOI] [PubMed] [Google Scholar]
- Kornak U, Reynders E, Dimopoulou A, van Reeuwijk J, Fischer B, Rajab A, Budde B, Nurnberg P, Foulquier F, Lefeber D, et al. 2008. Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet 40: 32–34 [DOI] [PubMed] [Google Scholar]
- Kornfeld S 1992. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem 61: 307–330 [DOI] [PubMed] [Google Scholar]
- Kozma K, Keusch JJ, Hegemann B, Luther KB, Klein D, Hess D, Haltiwanger RS, Hofsteenge J 2006. Identification and characterization of aβ1,3-glucosyltransferase that synthesizes the Glc-β1,3-Fuc disaccharide on thrombospondin type 1 repeats. J Biol Chem 281: 36742–36751 [DOI] [PubMed] [Google Scholar]
- Kranz C, Ng BG, Sun L, Sharma V, Eklund EA, Miura Y, Ungar D, Lupashin V, Winkel RD, Cipollo JF, et al. 2007. COG8 deficiency causes new congenital disorder of glycosylation type IIh. Hum Mol Genet 16: 731–741 [DOI] [PubMed] [Google Scholar]
- Krawitz PM, Schweiger MR, Rodelsperger C, Marcelis C, Kolsch U, Meisel C, Stephani F, Kinoshita T, Murakami Y, Bauer S, et al. 2010. Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat Genet 42: 827–829 [DOI] [PubMed] [Google Scholar]
- Lang L, Takahashi T, Tang J, Kornfeld S 1985. Lysosomal enzyme phosphorylation in human fibroblasts. Kinetic parameters offer a biochemical rationale for two distinct defects in the uridine diphospho-N-acetylglucosamine: lysosomal enzyme precursor N-acetylglucosamine-1-phosphotransferase. J Clin Invest 76: 2191–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang MR, Lapierre LA, Frotscher M, Goldenring JR, Knapik EW 2006. Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat Genet 38: 1198–1203 [DOI] [PubMed] [Google Scholar]
- Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE 2005. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab 90: 2424–2427 [DOI] [PubMed] [Google Scholar]
- Laufman O, Kedan A, Hong W, Lev S 2009. Direct interaction between the COG complex and the SM protein, Sly1, is required for Golgi SNARE pairing. EMBO J 28: 2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JA, Yip CK, Walz T, Hughson FM 2010. Molecular organization of the COG vesicle tethering complex. Nat Struct Mol Biol 17: 1292–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard-Melief C, Haltiwanger RS 2010. O-fucosylation of thrombospondin type 1 repeats. Methods Enzymol 480: 401–416 [DOI] [PubMed] [Google Scholar]
- Lesnik Oberstein SA, Kriek M, White SJ, Kalf ME, Szuhai K, den Dunnen JT, Breuning MH, Hennekam RC 2006. Peters plus syndrome is caused by mutations in B3GALTL, a putative glycosyltransferase. Am J Hum Genet 79: 562–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S 2006. Lipid homoeostasis and Golgi secretory function. Biochem Soc Trans 34: 363–366 [DOI] [PubMed] [Google Scholar]
- Li H, Yamagata T, Mori M, Momoi MY 2002. Association of autism in two patients with hereditary multiple exostoses caused by novel deletion mutations of EXT1. J Hum Genet 47: 262–265 [DOI] [PubMed] [Google Scholar]
- Lin X, Wei G, Shi Z, Dryer L, Esko JD, Wells DE, Matzuk MM 2000. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol 224: 299–311 [DOI] [PubMed] [Google Scholar]
- Lindahl U 1966. Further characterization of the heparin-protein linkage region. Biochim Biophys Acta 130: 368–382 [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Phair RD 2010. Lipids and cholesterol as regulators of traffic in the endomembrane system. Annu Rev Biophys 39: 559–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Xu YX, Hirschberg CB 2010. The role of nucleotide sugar transporters in development of eukaryotes. Semin Cell Dev Biol 21: 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E, Platt FM 2010. Lipids on trial: The search for the offending metabolite in Niemann-Pick type C disease. Traffic 11: 419–428 [DOI] [PubMed] [Google Scholar]
- Lonnqvist L, Karttunen L, Rantamaki T, Kielty C, Raghunath M, Peltonen L 1996. A point mutation creating an extra N-glycosylation site in fibrillin-1 results in neonatal Marfan syndrome. Genomics 36: 468–475 [DOI] [PubMed] [Google Scholar]
- Lowe JB 2005. Cell biology. Does Notch take the sweet road to success? Science 307: 1570–1572 [DOI] [PubMed] [Google Scholar]
- Lubbehusen J, Thiel C, Rind N, Ungar D, Prinsen BH, de Koning TJ, van Hasselt PM, Korner C 2010. Fatal outcome due to deficiency of subunit 6 of the conserved oligomeric Golgi complex leading to a new type of congenital disorders of glycosylation. Hum Mol Genet 19: 3623–3633 [DOI] [PubMed] [Google Scholar]
- Luo Y, Nita-Lazar A, Haltiwanger RS 2006. Two distinct pathways for O-fucosylation of epidermal growth factor-like or thrombospondin type 1 repeats. J Biol Chem 281: 9385–9392 [DOI] [PubMed] [Google Scholar]
- Maccioni HJ 2007. Glycosylation of glycolipids in the Golgi complex. J Neurochem 103: 81–90 [DOI] [PubMed] [Google Scholar]
- Maeda Y, Watanabe R, Harris CL, Hong Y, Ohishi K, Kinoshita K, Kinoshita T 2001. PIG-M transfers the first mannose to glycosylphosphatidylinositol on the lumenal side of the ER. EMBO J 20: 250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Ide T, Koike M, Uchiyama Y, Kinoshita T 2008. GPHR is a novel anion channel critical for acidification and functions of the Golgi apparatus. Nat Cell Biol 10: 1135–1145 [DOI] [PubMed] [Google Scholar]
- Marquardt T, Luhn K, Srikrishna G, Freeze HH, Harms E, Vestweber D 1999. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 94: 3976–3985 [PubMed] [Google Scholar]
- Martin PT 2007. Congenital muscular dystrophies involving the O-mannose pathway. Curr Mol Med 7: 417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Duncker I, Dupre T, Piller V, Piller F, Candelier JJ, Trichet C, Tchernia G, Oriol R, Mollicone R 2005. Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood 105: 2671–2676 [DOI] [PubMed] [Google Scholar]
- Matsuura H, Takio K, Titani K, Greene T, Levery SB, Salyan ME, Hakomori S 1988. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6. Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J Biol Chem 263: 3314–3322 [PubMed] [Google Scholar]
- Miyake N, Kosho T, Mizumoto S, Furuichi T, Hatamochi A, Nagashima Y, Arai E, Takahashi K, Kawamura R, Wakui K, et al. 2010. Loss-of-function mutations of CHST14 in a new type of Ehlers–Danlos syndrome. Hum Mutat 31: 966–974 [DOI] [PubMed] [Google Scholar]
- Morava E, Zeevaert R, Korsch E, Huijben K, Wopereis S, Matthijs G, Keymolen K, Lefeber DJ, De Meirleir L, Wevers RA 2007. A common mutation in the COG7 gene with a consistent phenotype including microcephaly, adducted thumbs, growth retardation, VSD and episodes of hyperthermia. Eur J Hum Genet 15: 638–645 [DOI] [PubMed] [Google Scholar]
- Morava E, Lefeber DJ, Urban Z, de Meirleir L, Meinecke P, Gillessen Kaesbach G, Sykut-Cegielska J, Adamowicz M, Salafsky I, Ranells J, et al. 2008. Defining the phenotype in an autosomal recessive cutis laxa syndrome with a combined congenital defect of glycosylation. Eur J Hum Genet 16: 28–35 [DOI] [PubMed] [Google Scholar]
- Morava E, Guillard M, Lefeber DJ, Wevers RA 2009. Autosomal recessive cutis laxa syndrome revisited. Eur J Hum Genet 17: 1099–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni F, Torelli S, Brockington M 2008. Muscular dystrophies due to glycosylation defects. Neurotherapeutics 5: 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng BG, Kranz C, Hagebeuk EE, Duran M, Abeling NG, Wuyts B, Ungar D, Lupashin V, Hartdorff CM, Poll-The BT, Freeze HH 2007. Molecular and clinical characterization of a Moroccan Cog7 deficient patient. Mol Genet Metab 91: 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SB, Turner EH, Robertson PD, Flygare SD, Bigham AW, Lee C, Shaffer T, Wong M, Bhattacharjee A, Eichler EE, et al. 2009. Targeted capture and massively parallel sequencing of 12 human exomes. Nature 461: 272–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng BG, Sharma V, Sun L, Loh E, Hong W, Tay SK, Freeze HH 2011. Identification of the first COG-CDG patient of Indian origin. Mol Genet Metab 102: 364–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Au CE, Bergeron JJ 2009. Sorting out glycosylation enzymes in the Golgi apparatus. FEBS Lett 583: 3764–3769 [DOI] [PubMed] [Google Scholar]
- Nishio M, Kamiya Y, Mizushima T, Wakatsuki S, Sasakawa H, Yamamoto K, Uchiyama S, Noda M, McKay AR, Fukui K, et al. 2010. Structural basis for the cooperative interplay between the two causative gene products of combined factor V and factor VIII deficiency. Proc Natl Acad Sci 107: 4034–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima T, Fukumoto S, Furukawa K, Urano T 1999. Molecular basis for the progeroid variant of Ehlers–Danlos syndrome. Identification and characterization of two mutations in galactosyltransferase I gene. J Biol Chem 274: 28841–28844 [DOI] [PubMed] [Google Scholar]
- Opat AS, Houghton F, Gleeson PA 2000. Medial Golgi but not late Golgi glycosyltransferases exist as high molecular weight complexes. Role of luminal domain in complex formation and localization. J Biol Chem 275: 11836–11845 [DOI] [PubMed] [Google Scholar]
- Paesold-Burda P, Maag C, Troxler H, Foulquier F, Kleinert P, Schnabel S, Baumgartner M, Hennet T 2009. Deficiency in COG5 causes a moderate form of congenital disorders of glycosylation. Hum Mol Genet 18: 4350–4356 [DOI] [PubMed] [Google Scholar]
- Peanne R, Legrand D, Duvet S, Mir AM, Matthijs G, Rorher J, Foulquier F 2010. Differential effects of lobe a and lobe b of the cog complex on the stability of b4galt1 and st6gal1. Glycobiology. [DOI] [PubMed] [Google Scholar]
- Percival JM, Froehner SC 2007. Golgi complex organization in skeletal muscle: A role for Golgi-mediated glycosylation in muscular dystrophies? Traffic 8: 184–194 [DOI] [PubMed] [Google Scholar]
- Pinhal MA, Smith B, Olson S, Aikawa J, Kimata K, Esko JD 2001. Enzyme interactions in heparan sulfate biosynthesis: uronosyl 5-epimerase and 2-O-sulfotransferase interact in vivo. Proc Natl Acad Sci 98: 12984–12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin E, Gladen A, Roden L, Kresse H 1990. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc Natl Acad Sci 87: 1342–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath M, Kielty CM, Steinmann B 1995. Truncated profibrillin of a Marfan patient is of apparent similar size as fibrillin: Intracellular retention leads to over-N-glycosylation. J Mol Biol 248: 901–909 [DOI] [PubMed] [Google Scholar]
- Ramirez IB, Lowe M 2009. Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol 20: 770–779 [DOI] [PubMed] [Google Scholar]
- Rasmussen H 1975. Isolated mammalian renal tubules. Methods Enzymol 39: 11–20 [DOI] [PubMed] [Google Scholar]
- Reitman ML, Varki A, Kornfeld S 1981. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5′-diphosphate-N-acetylglucosamine: Glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest 67: 1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynders E, Foulquier F, Leao Teles E, Quelhas D, Morelle W, Rabouille C, Annaert W, Matthijs G 2009. Golgi function and dysfunction in the first COG4-deficient CDG type II patient. Hum Mol Genet 18: 3244–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BC, Smith RD, Ungar D, Nakamura A, Jeffrey PD, Lupashin VV, Hughson FM 2009. Structural basis for a human glycosylation disorder caused by mutation of the COG4 gene. Proc Natl Acad Sci 106: 13329–13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts LM, Dlugosz M, Luther KB, Haltiwanger RS, Majerus EM 2007. O-fucosylation is required for ADAMTS13 secretion. J Biol Chem 282: 17014–17023 [DOI] [PubMed] [Google Scholar]
- Rivier AS, Castillon GA, Michon L, Fukasawa M, Romanova-Michaelides M, Jaensch N, Hanada K, Watanabe R 2010. Exit of GPI-anchored proteins from the ER differs in yeast and mammalian cells. Traffic 11: 1017–1033 [DOI] [PubMed] [Google Scholar]
- Roach JC, Glusman G, Smit AF, Huff CD, Hubley R, Shannon PT, Rowen L, Pant KP, Goodman N, Bamshad M, et al. 2010. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 328: 636–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S, et al. 2009. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol 11: 1197–1204 [DOI] [PubMed] [Google Scholar]
- Saroussi S, Nelson N 2009. Vacuolar H+-ATPase—An enzyme for all seasons. Pflugers Arch 457: 581–587 [DOI] [PubMed] [Google Scholar]
- Sato T, Sato M, Kiyohara K, Sogabe M, Shikanai T, Kikuchi N, Togayachi A, Ishida H, Ito H, Kameyama A, et al. 2006. Molecular cloning and characterization of a novel human β1,3-glucosyltransferase, which is localized at the endoplasmic reticulum and glucosylates O-linked fucosylglycan on thrombospondin type 1 repeat domain. Glycobiology 16: 1194–1206 [DOI] [PubMed] [Google Scholar]
- Schachter H, Jaeken J 1999. Carbohydrate-deficient glycoprotein syndrome type II. Biochim Biophys Acta 1455: 179–192 [DOI] [PubMed] [Google Scholar]
- Schmale GA, Conrad EU, 3rd, Raskind WH 1994. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am 76: 986–992 [DOI] [PubMed] [Google Scholar]
- Schwarz K, Iolascon A, Verissimo F, Trede NS, Horsley W, Chen W, Paw BH, Hopfner KP, Holzmann K, Russo R, et al. 2009. Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Genet 41: 936–940 [DOI] [PubMed] [Google Scholar]
- Simpson MA, Cross H, Proukakis C, Priestman DA, Neville DC, Reinkensmeier G, Wang H, Wiznitzer M, Gurtz K, Verganelaki A, et al. 2004. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet 36: 1225–1229 [DOI] [PubMed] [Google Scholar]
- Smith RD, Willett R, Kudlyk T, Pokrovskaya I, Paton AW, Paton JC, Lupashin VV 2009. The COG complex, Rab6 and COPI define a novel Golgi retrograde trafficking pathway that is exploited by SubAB toxin. Traffic 10: 1502–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Bolton AD, Funari V, Hong M, Boyden ED, Lu L, Manning DK, Dwyer ND, Moran JL, Prysak M, et al. 2010. Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N Engl J Med 362: 206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohda M, Misumi Y, Yoshimura S, Nakamura N, Fusano T, Ogata S, Sakisaka S, Ikehara Y 2007. The interaction of two tethering factors, p115 and COG complex, is required for Golgi integrity. Traffic 8: 270–284 [DOI] [PubMed] [Google Scholar]
- Spreafico M, Peyvandi F 2009. Combined Factor V and Factor VIII deficiency. Semin Thromb Hemost 35: 390–399 [DOI] [PubMed] [Google Scholar]
- Sprong H, Degroote S, Nilsson T, Kawakita M, Ishida N, van der Sluijs P, van Meer G 2003. Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol Biol Cell 14: 3482–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg SM, LaPointe P, Razvi A, Gurkan C, Potter CS, Carragher B, Balch WE 2008. Structural basis for cargo regulation of COPII coat assembly. Cell 134: 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P 2011. Golgi glycosylation. Cold Spring Harb Perspect Biol 3: a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Cummings R 2009. Structures common to different glycans. In Essentials of glycobiology, 2nd ed. (ed. Varki A, et al. ), pp. 175–198 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- Stickens D, Zak BM, Rougier N, Esko JD, Werb Z 2005. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 132: 5055–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara K, Schwartz NB 1979. Defect in 3′-phosphoadenosine 5′-phosphosulfate formation in brachymorphic mice. Proc Natl Acad Sci 76: 6615–6618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E, Lupashin V 2009. Role of vesicle tethering factors in the ER-Golgi membrane traffic. FEBS Lett 583: 3770–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, Kitani T, Kinoshita T 1993. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 73: 703–711 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Haltiwanger RS 2010. Role of glycosylation of Notch in development. Semin Cell Dev Biol 21: 638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, et al. 2004. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36: 579–581 [DOI] [PubMed] [Google Scholar]
- Torossi T, Guhl B, Roth J, Ziak M 2010. Endomannosidase undergoes phosphorylation in the Golgi apparatus. Glycobiology 20: 55–61 [DOI] [PubMed] [Google Scholar]
- Tucker RP 2004. The thrombospondin type 1 repeat superfamily. Int J Biochem Cell Biol 36: 969–974 [DOI] [PubMed] [Google Scholar]
- Turunen M, Schedin-Weiss S 2007. Defect in fatty acid esterification of dolichol in Niemann–Pick type C1 mouse livers in vivo. Biochim Biophys Acta 1771: 506–513 [DOI] [PubMed] [Google Scholar]
- ul Haque MF, King LM, Krakow D, Cantor RM, Rusiniak ME, Swank RT, Superti-Furga A, Haque S, Abbas H, Ahmad W, et al. 1998. Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse. Nat Genet 20: 157–162 [DOI] [PubMed] [Google Scholar]
- Ungar D 2009. Golgi linked protein glycosylation and associated diseases. Semin Cell Dev Biol 20: 762–769 [DOI] [PubMed] [Google Scholar]
- Urano Y, Watanabe H, Murphy SR, Shibuya Y, Geng Y, Peden AA, Chang CC, Chang TY 2008. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proc Natl Acad Sci 105: 16513–16518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW 2008. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol 9: 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile E, Oka T, Ericsson M, Nakamura N, Krieger M 2006. Intra-Golgi distribution of the conserved oligomeric Golgi (COG) complex. Exp Cell Res 312: 3132–3141 [DOI] [PubMed] [Google Scholar]
- Vogt G, Chapgier A, Yang K, Chuzhanova N, Feinberg J, Fieschi C, Boisson-Dupuis S, Alcais A, Filipe-Santos O, Bustamante J, et al. 2005. Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat Genet 37: 692–700 [DOI] [PubMed] [Google Scholar]
- Vogt G, Vogt B, Chuzhanova N, Julenius K, Cooper DN, Casanova JL 2007. Gain-of-glycosylation mutations. Curr Opin Genet Dev 17: 245–251 [DOI] [PubMed] [Google Scholar]
- Volker C, De Praeter CM, Hardt B, Breuer W, Kalz-Fuller B, Van Coster RN, Bause E 2002. Processing of N-linked carbohydrate chains in a patient with glucosidase I deficiency (CDG type IIb). Glycobiology 12: 473–483 [DOI] [PubMed] [Google Scholar]
- Westerlund B, Slotte JP 2009. How the molecular features of glycosphingolipids affect domain formation in fluid membranes. Biochim Biophys Acta 1788: 194–201 [DOI] [PubMed] [Google Scholar]
- Wu X, Steet RA, Bohorov O, Bakker J, Newell J, Krieger M, Spaapen L, Kornfeld S, Freeze HH 2004. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med 10: 518–523 [DOI] [PubMed] [Google Scholar]
- Yakubenia S, Frommhold D, Scholch D, Hellbusch CC, Korner C, Petri B, Jones C, Ipe U, Bixel MG, Krempien R, et al. 2008. Leukocyte trafficking in a mouse model for leukocyte adhesion deficiency II/congenital disorder of glycosylation IIc. Blood 112: 1472–1481 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, et al. 2003. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci 100: 3445–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP 2010. O-mannosyl phosphorylation of α-dystroglycan is required for laminin binding. Science 327: 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WW Jr 2004. Organization of Golgi glycosyltransferases in membranes: Complexity via complexes. J Membr Biol 198: 1–13 [DOI] [PubMed] [Google Scholar]
- Yuen CT, Chai W, Loveless RW, Lawson AM, Margolis RU, Feizi T 1997. Brain contains HNK-1 immunoreactive O-glycans of the sulfoglucuronyl lactosamine series that terminate in 2-linked or 2,6-linked hexose (mannose). J Biol Chem 272: 8924–8931 [DOI] [PubMed] [Google Scholar]
- Zak BM, Crawford BE, Esko JD 2002. Hereditary multiple exostoses and heparan sulfate polymerization. Biochim Biophys Acta 1573: 346–355 [DOI] [PubMed] [Google Scholar]
- Zdebska E, Mendek-Czajkowska E, Ploski R, Woeniewicz B, Koscielak J 2002. Heterozygosity of CDAN II (HEMPAS) gene may be detected by the analysis of erythrocyte membrane glycoconjugates from healthy carriers. Haematologica 87: 126–130 [PubMed] [Google Scholar]
- Zeevaert R, Foulquier F, Cheillan D, Cloix I, Guffon N, Sturiale L, Garozzo D, Matthijs G, Jaeken J 2009a. A new mutation in COG7 extends the spectrum of COG subunit deficiencies. Eur J Med Genet 52: 303–305 [DOI] [PubMed] [Google Scholar]
- Zeevaert R, Foulquier F, Dimitrov B, Reynders E, Van Damme-Lombaerts R, Simeonov E, Annaert W, Matthijs G, Jaeken J 2009b. Cerebrocostomandibular-like syndrome and a mutation in the conserved oligomeric Golgi complex, subunit 1. Hum Mol Genet 18: 517–524 [DOI] [PubMed] [Google Scholar]