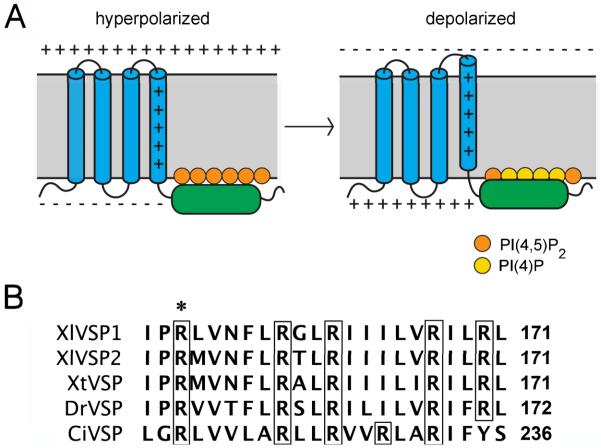
Fig. 1.
VSP transmembrane topology and voltage sensor sequence. A: The VSP protein is comprised of four transmembrane domains (blue) and a cytoplasmic phosphatase region (green). Depolarization-induced movement of the positively charged fourth transmembrane domain results in increased dephosphorylation of PI(4,5)P2, possibly due to movement of the phosphatase region closer to its substrate (see Murata and Okamura, 2007; Bezanilla, 2008; Villalba-Galea et al., 2009; Okamura and Dixon, 2011). B: Amino acids of the fourth transmembrane domain of VSPs of Xenopus laevis, Xenopus tropicalis, Danio rerio, and Ciona intestinalis. The transmembrane region is identified by the presence of multiple hydrophobic amino acids. The precise boundaries of this domain are predicted differently by different algorithms, and may vary depending on membrane potential. Arginines (R) are boxed, and R152 of Xl-VSP1 is indicated by an asterisk.