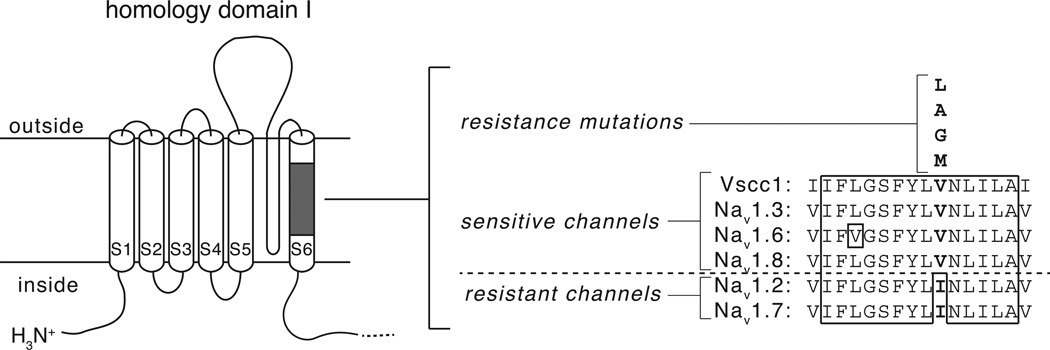
Fig. 7.
Identification of a sodium channel amino acid sequence polymorphism associated with differential pyrethroid sensitivity. Left: diagram of sodium channel homology domain I showing the location of a conserved block of amino acid sequence in transmembrane segment 6. Right: Aligned amino acid sequences in transmembrane segment 6 from four pyrethroid-sensitive channels (house fly Vssc1, rat Nav1.3, rat Nav1.6 and rat Nav1.8) and two pyrethroid-resistant channels (rat Nav1.2 and rat Nav1.7). Residues enclosed in the solid line are identical in all six sequences. Polymorphic residues aligning with V410 of Vssc1 are shown in boldface type; substitutions at the valine residue aligning with V410 of Vssc1 that are associated with pyrethroid resistance in insects are shown above the aligned sequences.