Table 1.
Receptor Affinities and Fluorescence Properties of Final Compounds
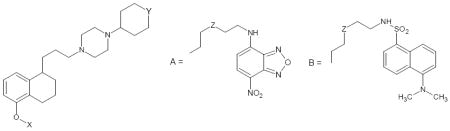 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki nMa | CHCl3 | EtOHb | PBSb,c | |||||||||||
| comp | X | Y | Z | σ1 | σ2 | λexc nm | λem nm | Φ | λexc nm | λem nm | Φ | λexc nm | λem nm | εd |
| 1e | CH3 | CH2 | 0.38±0.10 | 0.68±0.20 | ||||||||||
| 7 | CH3 | A | CH2CH2 | 2570f | 1720±160 | 450 | 515 | 0.17 | 476 | 520 | 0.08 | 480 | 535 | 14391 |
| 8 | CH3 | B | CH2CH2 | >5000f | 5020±180 | 346 | 490 | 0.32 | 335 | 507 | 0.29 | 340 | 510 | 2600 |
| 16 | A | CH2 | CH2CH2 | 78.7±18.2 | 10.8±3.0 | 451 | 514 | 0.20 | 467 | 520 | 0.05 | 460 | 520 | 11300 |
| 17 | A | CH2 | O | 96.2f | 39.3±11.8 | 450 | 512 | 0.18 | 465 | 520 | 0.04 | 460 | 520 | 6544 |
| 18 | B | CH2 | CH2CH2 | 9.08±1.32 | 20.8±1.5 | 340 | 490 | 0.30 | 335 | 507 | 0.20 | 345 | 485 | 4000 |
| 19 | B | CH2 | O | 19.8±8.7 | 25.7±4.7 | 345 | 490 | 0.48 | 335 | 507 | 0.23 | 343 | 510 | 2741 |
|
| ||||||||||||||
| (+)-pentazocine | 2.62±0.25 | |||||||||||||
| DTG | 24.6±2.2 | |||||||||||||
Values are the means of n ≥ 2 separate experiments.
Fluorescence properties herein reported were evaluated on compounds as free bases, but they were also evaluated on their corresponding hydrochloride salts in EtOH and PBS solutions. A maximum of 5 nm shift was observed in the excitation and emission wavelengths when compared to the excitation and emission wavelengths from the corresponding free bases.
All compounds solubilized in PBS gave Φ̣ value very close to 0 and therefore they are not reported.
From EtOH solutions of compounds in EtOH.
From Ref 18 where results from binding on human cells, in which compound 1 displays about 40-fold σ2 versus σ1 selectivity, are also reported.
From a unique experiment.
