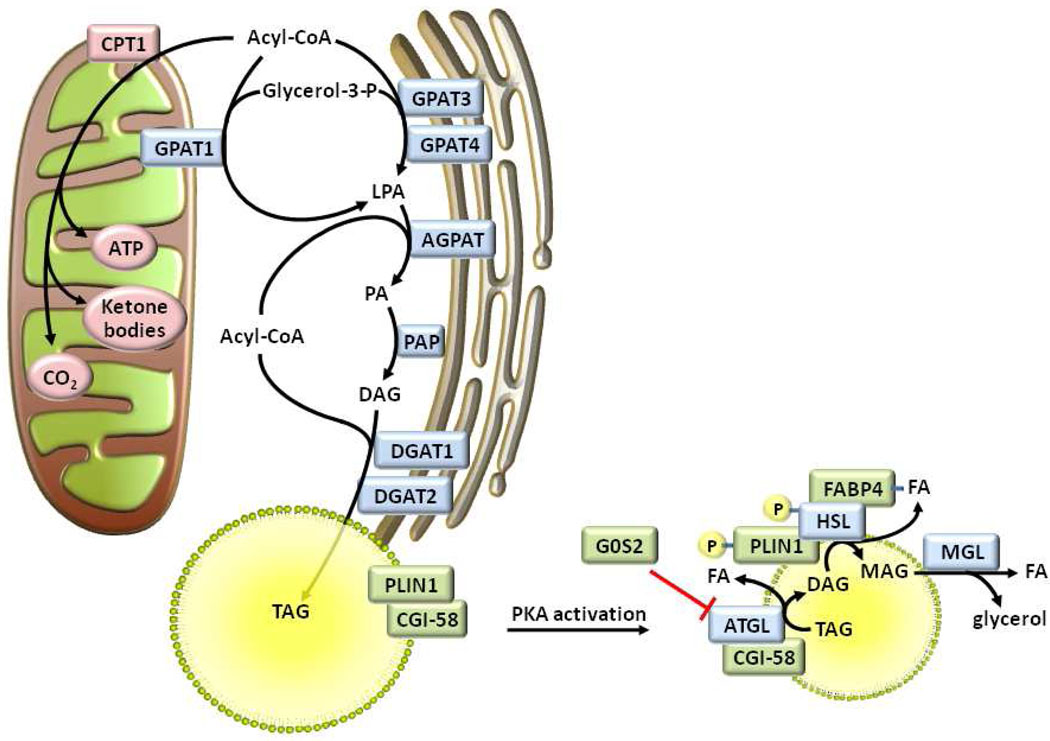
Figure 2. Integrated model of adipose TAG synthesis and lipolysis.
Acyl-CoAs are oxidized in mitochondria after they have been transported into the matrix as acyl-carnitines via carnitine palmitoyltransferase-1 (CPT1). Alternatively, acyl-CoAs may be esterified to glycerol-3-phosphate by a glycerol-3-phosphate acyltransferase (GPAT) isoform, resulting in the production of lysophosphatidic acid (LPA). One or more acyl-CoA:acylglycerol-3-phosphate acyltransferase (AGPAT) isoforms uses a second acyl-CoA to esterify the sn-2 position of LPA to form phosphatidic acid (PA). Phosphatidic acid phosphatase (PAP) dephosphorylates PA to form sn-1,2-diacylglycerol (DAG). Diacylglycerol acyltransferase (DGAT) isoenzymes use a final acyl-CoA to synthesize triacylglycerol (TAG) from DAG. Upon lipolytic stimulation and protein kinase A (PKA) activation, perilipin (PLIN1) is phosphorylated, thereby promoting the release of comparative gene identification-58 (CGI-58) which recruits hormone sensitive lipase (HSL) to the lipid droplet. CGI-58 binds adipose triglyceride lipase (ATGL), facilitates the translocation of ATGL to the lipid droplet, and promotes ATGL’s TAG hydrolytic activity. G0S2 inhibits ATGL, although its interaction with ATGL is not dependent upon PKA activation, as illustrated. PKA also phosphorylates HSL, thereby allowing it to move to the surface of the lipid droplet, and increasing its activity towards DAG. The interaction of HSL with fatty acid binding protein-4 (FABP4) promotes FA efflux from the lipid droplet and alleviates its product inhibition of HSL. Finally, monoacylglycerol lipase (MGL) hydrolyzes monoacylglycerol (MAG), releasing glycerol and a fatty acid (FA).