Figure 3.
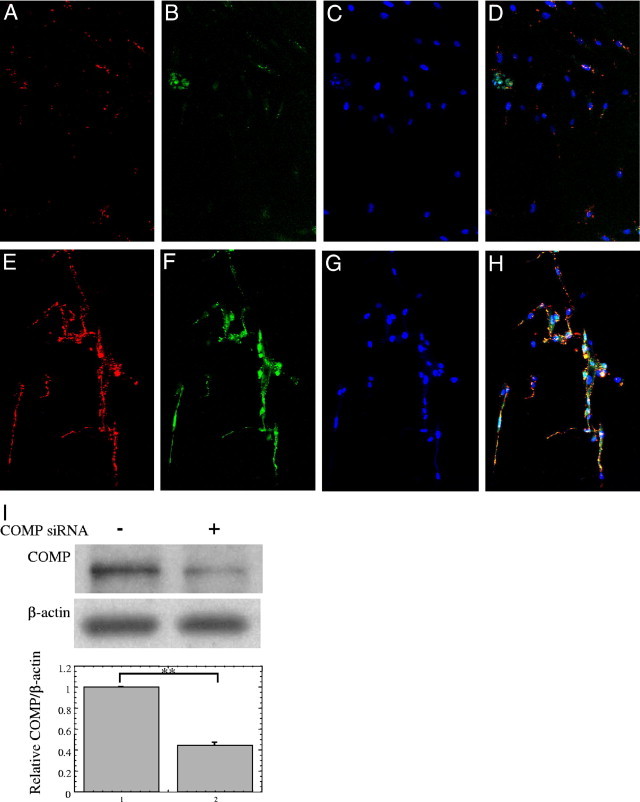
Suppression of cartilage oligomeric matrix protein (COMP) by small interfering RNA (siRNA) reduces levels of extracellular type I collagen. At 96 hours after transient transfection with the COMP siRNA (A–D) or with the control siRNA (E–H) into subconfluent keloid-derived fibroblasts (KDFs), the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Extracellular type I collagen was then detected with an anti-type I collagen antibody and was visualized using a red fluorophore (A and E); COMP was detected with a COMP-specific antibody and visualized using a green fluorophore (B and F), and nuclei were stained with Hoechst (C and G). A merged image (A, B, and C) is shown in (D) and an image (E, F, and G) is shown in (H). Transfection of the COMP siRNA reduces levels of extracellular type I collagen and COMP (A versus E, B versus F). Nuclei of fibroblasts were nonspecifically stained in each experiment. The distribution of type I collagen and COMP overlapped closely (H). The cells were observed by confocal laser scanning microscope and all photographs are shown at ×20 magnification. (I) KDFs transiently transfected with the control or COMP siRNA were lysed in 100 μL 2 × SDS-PAGE buffer in reducing conditions and subjected to Western blot for COMP and β-actin. The COMP/β-actin ratios were determined and expressed as the relative of COMP/β-actin of the each control sample transfected with control siRNA. The lower graph shows mean ± SD of COMP/β-actin protein ratios using the KDFs from three different patients (n = 3). Statistical difference was determined by the Student's t-test. **P < 0.01.
