Figure 3.
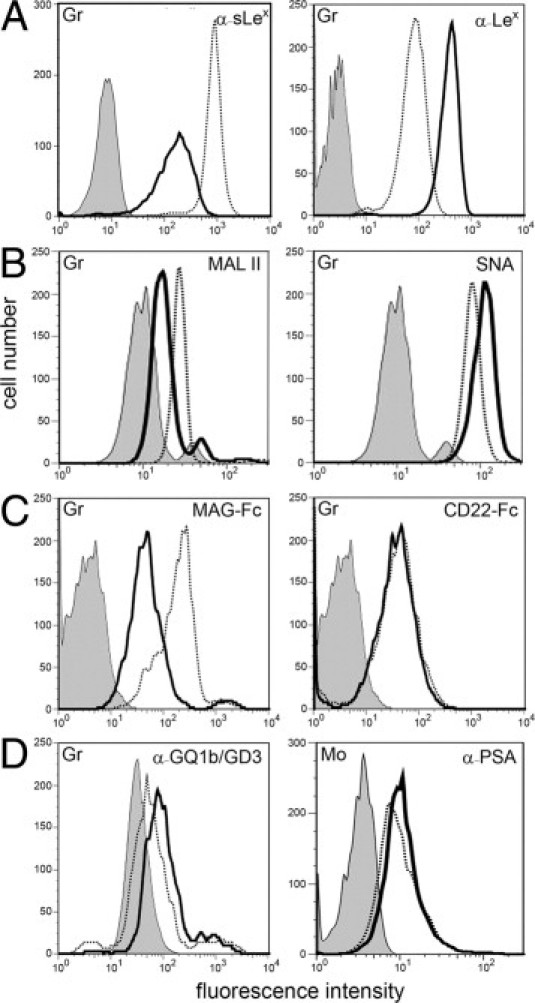
Myeloid cells display a defect in α2,3-sialylation. Granulocytes (Gr) were tested for binding of the following reagents: mAbs against sLex and Lex (A), Maackia amurensis lectin II (MAL II, binds preferentially to α2,3-sialylated structures) and Sambucus nigra lectin (SNA, binds preferentially to α2,6-sialylated structures) (B), myelin-associated glycoprotein-Fc chimera (MAG-Fc, Siglec-4-Fc; binds to α2,3-sialylated structures) and CD22-Fc (Siglec-2-Fc; binds to α2,6-sialylated structures) (C), and a mAb that detects α2,8-sialylated gangliosides GQ1b and GD3 (D, left panel). Binding of a mAb specific for α2,8-sialylated polysialic acid (PSA) to monocytes (Mo) is show in D (right panel). Background signals were obtained with isotype control mAbs (for mAbs), secondary reagent only (for lectins), and VE-cadherin-Fc (for Fc-constructs). Background signals in control and patient cells were virtually identical. The latter are shown. Results are representative for at least three experiments per panel.
