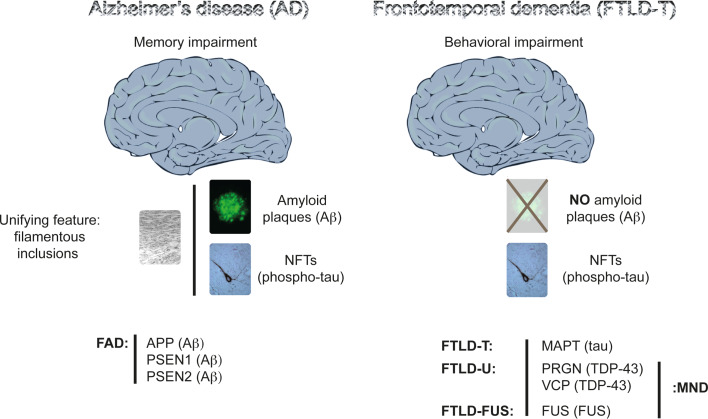
Fig. 1.
Histopathological and genetic features of Alzheimer’s disease (AD) and frontotemporal dementia (FTD). Memory impairment characterizes AD at a clinical level, and the presence of amyloid (Aβ) plaques and phospho-tau-containing neurofibrillary tangles (NFTs) in brain at a histopathological level. A unifying feature of the plaques and tangles is that their major proteinaceous components, Aβ and tau, respectively, are fibrillar. Plaques are scarce in FTD. The prominent feature in FTD is a behavioral impairment, with memory functions often being preserved until late in disease. Compared to AD, FTD is a highly heterogeneous group of related dementias, as reflected both by the function of the mutated genes, by the proteins that are deposited as insoluble aggregates, and by the clinical syndromes, with language and behavioral variants known. A subset of FTD, known as frontotemporal lobar degeneration with tau deposits (FTLD-T) or FTD with Parkinsonism linked to chromosome 17 (FTDP-17), is characterized by tau inclusions. The first FTD mutations were identified in the tau-encoding MAPT gene causing FTLD-T. Mutations have been subsequently identified in the PGRN gene encoding progranulin, and in the VCP gene encoding valosin-containing protein. TDP-43 is the deposited protein, and these deposits are shared with motor neuron disease (MND), also known as amyotrophic lateral sclerosis (ALS). Fused in sarcoma (FUS) is another pathological protein that has been identified in a small subset of patients with either ALS or a form of FTD. In familial AD (FAD), mutations have been identified in the APP gene encoding the amyloid precursor protein from which Aβ is derived by proteolytic cleavage, and in the genes encoding presenilin 1 and 2 (PSEN1 and PSEN2), which form part of the Aβ cleavage machinery. In AD, no mutations have been identified in the tau-encoding MAPT gene