Abstract
AIM: To demonstrate that CD14+ cells are an important source of the growth factor YKL-40 in acute and chronic liver damage.
METHODS: Rats were inoculated with one dose of CCl4 to induce acute damage. Liver biopsies were obtained at 0, 6, 12, 24, 48 and 72 h. For chronic damage, CCl4 was administered three days per week for 6 or 8 wk. Tissue samples were collected, and cellular populations were isolated by liver digestion and purified by cell sorting. YKL-40 mRNA and protein expression were evaluated by real-time polymerase chain reaction and western blot.
RESULTS: Acute liver damage induced a rapid increase of YKL-40 mRNA beginning at 12 h. Expression peaked at 24 h, with a 26-fold increase over basal levels. By 72 h however, YKL-40 expression levels had nearly returned to control levels. On the other hand, chronic damage induced a sustained increase in YKL-40 expression, with 7- and 9-fold higher levels at 6 and 8 wk, respectively. The pattern of YKL-40 expression in different subpopulations showed that CD14+ cells, which include Kupffer cells, are a source of YKL-40 after acute damage at 72 h [0.09 relative expression units (REU)] as well as after chronic injury at 6 wk (0.11 REU). Hepatocytes, in turn, accounted for 0.06 and 0.01 REU after 72 h (acute) or 6 wk (chronic), respectively. The rest of the CD14- cells (including T lymphocytes, B lymphocytes, natural killer and natural killer T cells) yielded 0.07 and 0.15 REU at 72 h and 6 wk, respectively. YKL-40 protein expression in liver was detected at 72 h as well as 6 and 8 wk, with the highest expression relative to controls (11-fold; P ≤ 0.05) seen at 6 wk. Macrophages were stimulated by lipopolysaccharide. We demonstrate that under these conditions, these cells showed maximum expression of YKL-40 at 12 h, with P < 0.05 compared with controls.
CONCLUSION: Hepatic CD14+ cells are an YKL-40 mRNA and protein source in acute and chronic liver injury, with expression patterns similar to growth factors implicated in inflammation-fibrogenesis.
Keywords: YKL-40, Kupffer cells, Liver cirrhosis, CD14+ cells
INTRODUCTION
YKL-40 glycoprotein (Chi3L1) is a growth factor named for its N-terminal amino acid sequence and molecular weight. It is related to 18-glycosylhydrolases, but lacks enzymatic activity[1,2]. Under physiological conditions, human YKL-40 is secreted in small quantities by synoviocytes and chondrocytes[3,4]. However, in situ experiments demonstrated increased tissue mRNA and protein levels in osteoarthritis and rheumatism as well as in patients with inflammatory joint disease, where activated monocytes and macrophages are the main source.
The biological function of YKL-40 is unclear because no receptors have been identified to date. However, it has been reported that YKL-40 binds to stabilin type 1 and heparin sulfate in vivo. Furthermore, YKL-40 stimulates the proliferation and migration of connective tissue cells and fibroblasts through activation of the mitogen-activated protein kinase signaling pathway, suggesting that YKL-40 modulates fibrogenesis and tissue remodeling[5,6].
Several studies have found elevated YKL-40 concentrations in sera of patients with liver diseases, such as hepatic fibrosis by hepatitis C virus[7]. Serum concentrations of YKL-40 correlated with extracellular matrix (ECM) products secreted by hepatic stellate cells (HSCs) and fibroblasts (e.g., PIIINP, hyaluronan, MMP-2, and TIMP-1). It has been suggested that YKL-40 concentrations reflect the degree of liver fibrosis. However, extensive clinical evaluation is still required, and other inflammatory diseases have to be excluded as potential causes of YKL-40 elevations.
In hepatic tissue, immunohistochemical analyses show strong YKL-40 staining around fibrotic areas[3]; however, in this study it was not possible to discriminate the cells that produce YKL-40. Notably, HSCs from fibrotic liver tissue by S. japonica showed an increase of YKL-40 mRNA[8].
Currently, the kinetics and source of YKL-40 in the liver under damage conditions are unknown. This study addressed these issues as part of a broader effort to elucidate the role of this molecule in hepatic inflammation and tissue repair.
MATERIALS AND METHODS
Animals
Rats were treated according to the guidelines for reproduction, care and use of laboratory animals stated by the Norma Oficial Mexicana (NOM-066-ZOO-1999). Twenty-seven male Wistar rats weighing 250 g each were selected for treatment with CCl4 and divided into nine groups of three animals each. One group was untreated and used as controls. Six groups were inoculated with a single intragastric dose of 0.5 mL/100 g of CCl4 (Sigma, 319961, United States) mixed 1:1 with mineral oil (Sigma, M5409, United States) to establish acute injury. These animals were sacrificed at time 0, 6, 12, 24, 48 and 72 h. To establish chronic liver injury, two groups were inoculated three times per week with an i.p. injection of 0.1 mL/100 g CCl4: mineral oil at ratios of 1:6, 1:5, 1:4 (one week for each), then 1:3 until the animals were sacrificed at week 6 or 8.
Tissue samples
Procedures were performed under ether anesthesia. Livers were washed with PBS (Gibco, 70013-032, United Kingdom) at 4 °C, sectioned into small 100 mg pieces and stored in tubes at -70 °C. Samples for RNA extraction were stored in 500 μL of TRIzol (Invitrogen® BRL 15596-026, United States) at -70 °C.
RNA extraction
Liver tissue and cells were homogenized in 500 μL of TRIzol (Invitrogen® BRL United States). Chloroform (Sigma, C2432, United States) was added, and the samples were centrifuged for 15 min at 10 000 r/min and 4 °C. Total RNA was precipitated with isopropanol (Sigma, 19516, United States). The RNA pellet was washed with 75% ethanol and dissolved in RNase-free water. The final concentrations and quality of the RNA were determined by spectrophotometry.
Reverse-transcriptase polymerase chain reaction
Approximately 1 μg of total RNA was reverse-transcribed to cDNA in a 20 μL reaction using murine leukemia virus reverse transcriptase M-MLV (Invitrogen®BRL, 28025-013, United States). The mixture was prepared with 1 μg of RNA, 125 ng/μL of random primers (Invitrogen® BRL, 48190011, United States), 1 μL of 10 mmol/L dNTP mix and 12 μL of sterile distilled water. The mixture was heated for 5 min at 65 °C and quick chilled on ice. Four μL of 5X First Strand Buffer, 2 μL of 0.1 mol/L DTT and 1 μL of RNaseOUT (Invitrogen® BRL, 10777019, United States) were added, and the reaction was incubated at 37 °C for 2 min. Finally, 200 U/μL of M-MLV were added, and the total reaction was incubated at 25 °C for 10 min and then at 37 °C for 50 min. The reaction was inactivated by heating at 70 °C for 15 min[9].
Real–time polymerase chain reaction
Real-time polymerase chain reaction (PCR) was performed with an ABI Prism 7300 thermocycler (Applied Biosystems, United States). Each 20 μL reaction contained 2 μL of cDNA and TaqMan Universal PCR master mix (Applied Biosystems, 4364338, United States). The primers and probe set sequences were specific for 18S rRNA (constitutive gene; Applied Biosystems, FG18S RNA, United States) and YKL-40 (inducible gene; Applied Biosystems, Rn0149065, United States). All reactions were run in duplicate at universal thermocycler conditions for TaqMan® Gene Expression Assays (2 min at 50 °C, 10 min at 95 °C and 40 cycles at 95 °C for 15 s and 60 °C for 1 min). Results were analyzed with ABI Prism software[9].
YKL40 expression in cell subpopulations
Male Wistar rats were anesthetized, and livers were perfused via the portal vein with calcium-free Gey’s solution (Sigma, 69779, United States) supplemented with 100 U/mL of heparin. A second perfusion was done with calcium-free Gey’s solution containing 100 U/mL of heparin, 0.06% collagenase (Fluka, 27678, United States) and 0.01% DNase (Roche, 13035000, Sweden). Liver tissues were extracted, homogenized and placed in a bottle containing Gey’s solution, 0.005% collagenase and 0.001% DNase, and stirred gently for 30 min at 37 °C. The resultant suspension was filtered in 106 Nylon mesh, and 4 mL of MEM (Invitrogen® BRL, 11095, United States) supplemented with 10% fetal bovine serum (Invitrogen® BRL, 1082-139, United States) were added. The homogenates were centrifuged at 50 G for 2 min at 4 °C to precipitate hepatocytes. The supernatant was recovered and centrifuged at 400 G for 7 min at 4 °C. The pellet was resuspended with Gey’s solution plus 25% albumin (Sigma, A7906, United States) and centrifuged at 350 G for 10 min at 4 °C to obtain non-parenchymal cells (NPCs). NPCs were washed, resuspended with Gey’s solution, placed on a lymphoprep (Axis-Shield, LYS3773, Oslo, Denmark) gradient and centrifuged at 1800 r/min for 25 min. The white ring of mononuclear cells was recovered. Cells were washed and then divided into aliquots of 1 × 106 per tube for sorting. Next, cells were incubated with a CD14 primary antibody (Santa Cruz Biotechnology, M305, United States, 1:25) for 30 min in the dark at 4 °C. Cells were then washed and incubated with a FITC secondary antibody (Jackson ImmunoResearch, 71813, United Kingdom, 1:2000) for 30 min at 4 °C in the dark. Marked cells were washed and sorted (BD FACSAria) by CD14 protein surface marker expression.
Western blotting
Protein extraction from whole liver tissue and cellular populations was performed with the NE-PER (Pierce, 78833, United States) extraction reagent according to the manufacturer’s instructions. Protein concentrations were quantified with the BioRad protein assay (BioRad, 500-13, 14, 15; United States). Sodium dodecyl sulfate polyacrylamide gel electrophoresis was done at 12%, and the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane at 30 V overnight at 8 °C. The PVDF membrane was washed twice in tris buffered saline (TBS) and blocked with 5% of non-fat milk used for 1 h at room temperature with constant stirring. The membrane was then incubated for 1 h at room temperature with primary antibodies against YKL-40 (Santa Cruz Biotechnology, sc-31722, United States, 1:200) or [-Actin actin (Santa Cruz Biotechnology, sc-47778, United States, 1:500)]. Membranes were then washed twice with tris buffered saline Tween-20 (TBST) and incubated for 30 min with an HRP-conjugated donkey anti-goat secondary antibody (Santa Cruz Biotechnology, sc-2020, United States, 1:4000) to detect YKL-40 or goat anti-mouse for [-actin (Roche, 11520709001, Sweden, 1:500)]. Membranes were then washed twice in TBST and TBS. The signal was detected with the BM chemiluminescence Western Blotting kit (Roche, 1520709, Sweden) according to the manufacturer’s instructions. Relative expression units (REU) were calculated from densitometric values of YKL-40 and β-actin (Kodak MI SE 4.5 software, Kodak, United States).
In vitro overexpression of YKL-40
Alveolar macrophage cells (ATCC, NR8383) were cultured in RPMI 1640 medium (Invitrogen® BRL, 21870084, United States) supplemented with 10% fetal calf serum and 1% antibiotic-antimycotic (Invitrogen® BRL, 15240062, United States). Upon reaching 70% confluence, cells were stimulated with LPS (Sigma, L4005, United States, 100 ng/L) for 3 h or 12 h; the medium was then discarded, and the cells were lysed in TrizolTRIzol reagent for mRNA extraction and evaluation of YKL-40 mRNA expression by real-time PCR.
Statistical analysis
The statistical analysis was performed with SPSS 10.0 software (SPSS Inc., United States), and significance was calculated by ANOVA. Data were expressed as mean ± SD. We considered P value ≤ 0.05 to be significant.
RESULTS
YKL-40 mRNA expression
Real-time PCR was performed on liver tissue samples after acute or chronic injury to evaluate changes in YKL-40 mRNA expression. In all experiments, healthy animals served as the control group. At 6 and 12 h after CCl4 administration, YKL-40 expression was increased 2- and 10-fold, respectively, compared to controls (P ≤0.05). Maximum expression (26-fold) relative to healthy tissue samples occurred at 24 h. At 72 h, YKL-40 mRNA expression levels declined to a level close to that of the 0-h and healthy groups (Figure 1).
Figure 1.
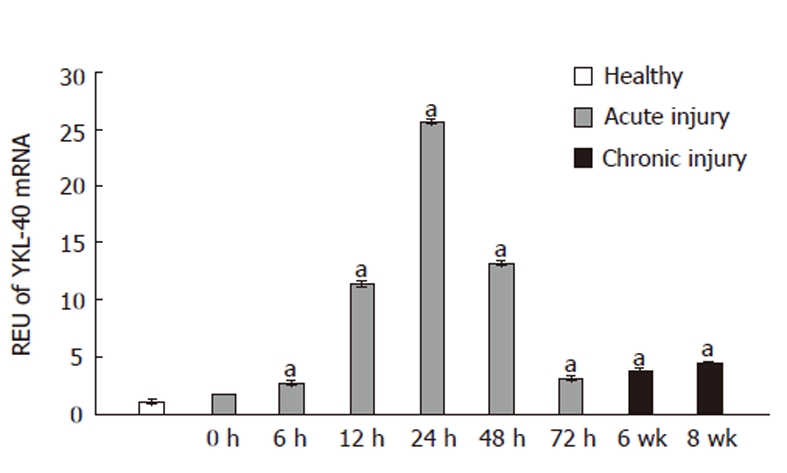
YKL-40 mRNA expression in whole liver tissue samples with acute and chronic injuries induced by CCl4. After acute injury, YKL-40 mRNA increased in a gradual and constant pattern and then fell to control levels. The maximum 26-fold increase was reached at 24 h in the acute damage model. For the chronic injury model, at 6 and 8 wk, YKL-40 mRNA levels were increased 7- and 9-fold compared to controls, respectively. aP ≤ 0.05. REU: Relative expression units.
In the chronic liver injury model, YKL-40 mRNA expression was significantly increased relative to controls at both 6 and 8 wk (8 and 10 REU, respectively, P < 0.05) (Figure 1).
YKL-40 protein expression
We next investigated the presence of YKL-40 protein in whole liver tissue from healthy, acute and chronic injury samples. In livers with acute injury, we observed that YKL-40 protein levels peaked at 72 h, with an increase of 2.0-fold compared to controls. At 12 and 24 h, YKL-40 levels were similar to those of healthy livers. In contrast, in the chronic injury model the maximum peak was observed at 6 wk with a significant decline apparent by 8 wk (P < 0.05) (Figure 2).
Figure 2.
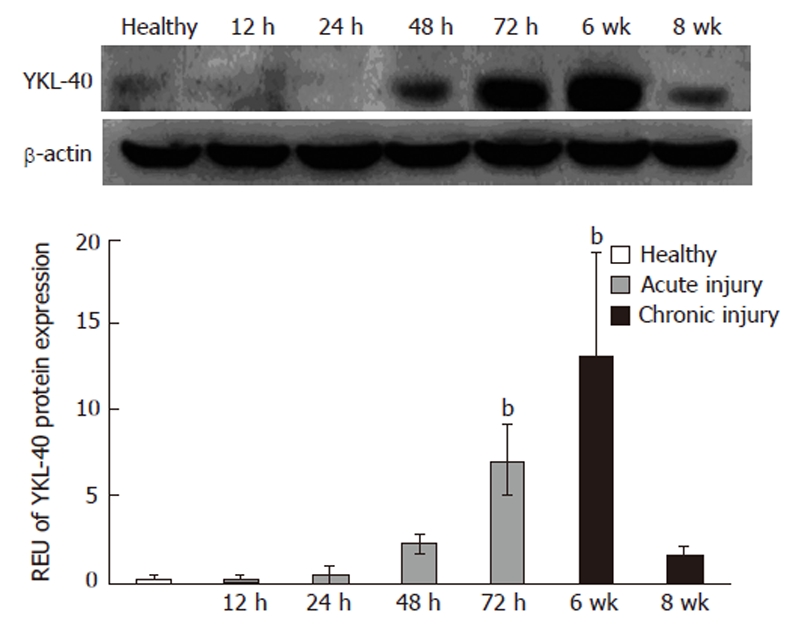
YKL-40 protein levels show a gradual increase. The maximum peak was at 72 h in the acute damage model and 6 wk in the chronic injury model, with 2.01 and 6.35-fold increases, respectively. bP ≤ 0.05 respect to the control group. REU: Relative expression units.
CD14+ cells are a source of YKL-40 protein
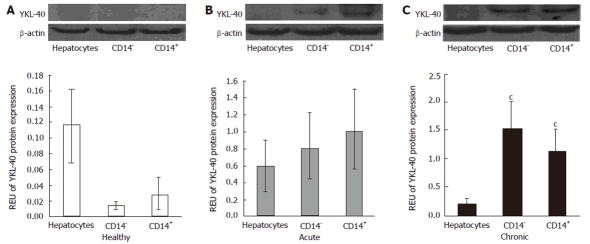
Based on their high levels of YKL-40 protein expression in whole liver samples, the 72-h group from the acute model and the 6-wk group from the chronic model were chosen for evaluation of protein expression in isolated cells. It has been reported that CD14+ cells population, which includes Kupffer macrophages, synthesize the largest amount of cytokines and growth factors. Additionally, they are found in abundance in the liver, accounting for about 20%-25% of NPCs[10] and 80%-90% of macrophages in the whole body. Therefore, we evaluated YKL-40 protein expression in this subpopulation.
Hepatocytes, CD14+ cells and CD14- cells isolated from healthy animals did not show significant YKL-40 protein expression, with no statistical significance between groups (Figure 3A). However, when these cells types were isolated from CCl4-treated animals, YKL-40 protein expression was elevated up to 4-fold compared with healthy animals. The highest level (0.09 REU) was detected in CD14+ cells in the acute damage model (Figure 3B). In the chronic injury model, both CD14- and CD14+ cells were significant sources of YKL-40, with 0.15 and 0.11 REU, respectively (P < 0.05 compared to control cells) (Figure 3C).
Figure 3.
YKL-40 protein expression in cellular populations isolated from liver tissue. A: In healthy livers, expression of YKL-40 protein was lower in CD14+ and CD14- subpopulations. B: In cells from livers exposed to acute damage, YKL-40 protein levels were higher in CD14+ cells. C: In the chronic injury model, hepatocytes showed low levels of YKL-40, with significant difference between CD14+ and CD14- cells against hepatocytes (cP < 0.05). REU: Relative expression units.
Lipopolysaccharide induces YKL-40 mRNA expression in rat alveolar macrophages
Previous studies reported that CCl4 induces damage to the intestinal tissue architecture[11]. CCl4 promotes translocation of bacteria and associated compounds like Lipopolysaccharide (LPS) towards the liver by portal blood flow. Here, LPS is taken up by Kupffer cells, which then synthesize cytokines and growth factors. To demonstrate this effect, rat alveolar macrophage cells were stimulated with LPS for 3 or 12 h. Our results showed that YKL-40 mRNA levels were increased 9- and 11-fold, respectively, compared with control cells without LPS stimulation (P ≤ 0.05). However, no significant difference was detected between the 3- and 12-h groups (Figure 4).
Figure 4.
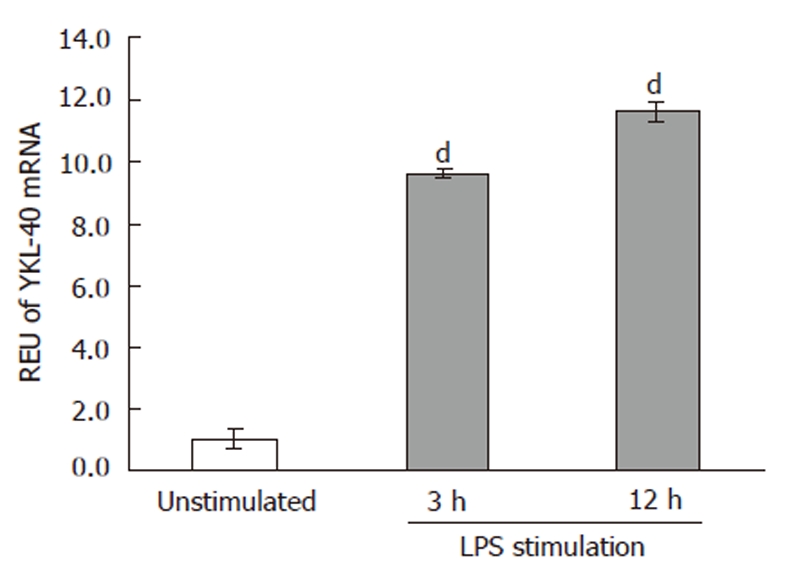
YKL-40 mRNA expression was induced by lipopolysaccharide in cell culture. After 3 and 12 h of stimulation with lipopolysaccharide, YKL-40 mRNA levels were increased by 9- and 11-fold, respectively, in a rat alveolar macrophage cell line (dP < 0.05). Unstimulated cells were used as controls. REU: Relative expression units. LPS: Lipopolysaccharide.
DISCUSSION
Several studies have found a correlation between serum YKL-40 levels and liver fibrosis stages[10,12,13]. However, other studies have reported contradictory findings[14-18]. Nevertheless, in the first set of studies, procollagen IIIpeptide and hyaluronic acid showed better correlations with fibrosis than YKL-40, likely because these molecules are scar components. YKL-40 is presumably a growth factor that indirectly contributes to fibrosis by stimulating proliferation of the cells that produce ECM proteins. For this reason, we considered it important to study the expression kinetics and source of YKL-40 in models of acute and chronic liver injury.
Although liver fibrosis and cirrhosis are characterized by inflammatory infiltration, a process in which a great number of cells participate, Johansen et al[3] showed that the liver was a possible source of YKL-40. This study noted that strong YKL-40 immunostaining could be detected around fibrotic areas in liver tissue samples where fibrosis had been induced by alcohol and viral hepatitis, but it was impossible to distinguish the cellular source[3].
We used the CCl4 damage model because it resembles alcohol damage[14] and because the kinetics of damage is well characterized. Our results show that YKL-40 mRNA levels began to increase at 12 h, with a maximum peak at 24 h (Figure 1). In the CCl4 fibrosis model, increased mRNA levels of growth factors such as TGF-β and PDGF at 48 and 72 h after intoxication suggest their participation in the fibrogenic process through their biological activities of ECM synthesis and HSC-fibroblast proliferation respectively[15,19]. Like PDGFβ, YKL-40 might activate the proliferating cell signaling pathway PI3K-AKT[20].
Kupffer cells were the natural candidate source of YKL-40 because previous studies of joint, lung, kidney and skin inflammatory diseases[2,5] identified macrophages as the main producer of this protein. In our study, CD14+ cells were a source of YKL-40, exhibiting 0.9 and 0.11 REU in the acute and chronic damage models, respectively. However, it is important to note that CD14- cells also produced YKL-40 protein, with 0.7 and 0.15 REU in the acute and chronic injury models, respectively. This population includes immune cells such as natural killer (NK) and natural killer T (NKT) cells, which represent about 37% and 26% of non-parenchymal cells respectively. These cells are the first line of defense in liver infections and consequently have immunoregulatory properties: they can synthesize cytokines (e.g., IFN-γ, TNF-α, IL-4, IL-10 and IL-13) (NK cells) or induce direct cellular destruction by TLR or CD1D molecules (NKT cells)[11].
LPS might also be a trigger of YKL-40 gene expression. Carbon tetrachloride and alcohol injure the upper gastrointestinal tract, forming lesions in gastric and duodenal mucosa. This promotes increased intestinal permeability to endotoxins, notably LPS, peptidoglycan, flagellin and zymosan, which are found in liver blood influx as well. These antigens are taken up by monocytes and Kupffer cells[12,13], which express TLR 1-8. LPS is known to be a ligand for TLR-4, a toll-like receptor widely expressed in monocytes and macrophages. This ligand-receptor interaction induces larger quantities of TNF-α, TGF-β and IL-10 cytokines[12,13]. Considering that the type of intestinal and liver damage induced by CCl4 is similar to that of ethanol consumption, we stimulated alveolar macrophages in vitro with 100 ng/L and found increases in YKL-40 mRNA levels of about 9- and 12-fold at 3 and 12 h, respectively. These results indicate that LPS could act as a direct stimulus to induce YKL-40 in macrophages during the establishment of damage.
COMMENTS
Background
Hepatic diseases are a major cause of death in the world; some have, as a singular characteristic, the presence of fibrosis. Hepatic fibrosis is difficult to diagnose because a hepatic biopsy is needed, and the condition of patients frequently makes it impossible to perform a biopsy. For several years, researchers have been looking for fibrosis markers in the blood in order to avoid having to perform a hepatic biopsy. One of these is YKL-40, a protein produced by several tissues under conditions causing stress and damage, such as alcohol consumption. YKL-40 has been used as a fibrosis marker with controversial results, particularly since it is not known precisely which cells produce this protein.
Research frontiers
The focus of this article is to elucidate which cells in the liver are producing YKL-4 and to try to understand the role of YKL-40 in the process of repairing fibrotic hepatic tissue and the maintenance of a healthy liver.
Innovations and breakthroughs
Until the present moment, there has not been a study that describes in which cells and at what time YKL-40 is produced. Several research protocols in humans have been made: in 2003 Nojgaard detected YKL-40 and successfully related this protein with hepatic fibrosis, however, in recent years other research has shown contradictory results. Johansen in 2000 published that, in liver biopsies, cells were producing YKL-40; however with the methodology used it was impossible to distinguish which cells types were actively producing YKL-40. This work was initiated because the authors thought that it was necessary to know more about YKL-40 production and to focus on the hepatic cellular source.
Applications
The hepatic cells that produce YKL-40 are CD14+ and CD14- at different times. This helps the authors to better understand how serum YKL-40 could be increased at different stages of disease, and attempt to better understand YKL-40 as a blood fibrosis marker and how this molecule participates in the process of hepatic health and illness.
Terminology
YKL-40: Protein produced by cells that helps to augment cell growth and to stimulate proteins that form the extracellular environment. CD14: Molecules in the cell membrane useful to classify and distinguish cells.
Peer review
A research study about a model of hepatic injury in the rat that is relevant since nowadays liver cirrhosis/fibrosis is a health problem worldwide. Many treatment modalities have been suggested to prevent the development of fibrosis but, unfortunately, none of them have uniformly yielded promising results. Therefore, a better knowledge of the pathogenic mechanisms involved in the inflammatory response developed during acute and chronic liver diseases makes possible the use of more effective therapeutic approaches in these patients.
Footnotes
Supported by Complementary support CONACyT 90361
Peer reviewer: Maria-Angeles Aller, MD, PhD, Professor, Cátedra de Cirugía, Facultad de Medicina, Universidad Complutense de Madrid, Pza. de Ramón y Cajal s.n., Madrid 28040, Spain
S- Editor Sun H L- Editor Rutherford A E- Editor Li JY
References
- 1.De Ceuninck F, Gaufillier S, Bonnaud A, Sabatini M, Lesur C, Pastoureau P. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun. 2001;285:926–931. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi D, Podswiadek M, Zaninotto M, Punzi L, Plebani M. YKL-40 as a marker of joint involvement in inflammatory bowel disease. Clin Chem. 2003;49:1685–1688. doi: 10.1373/49.10.1685. [DOI] [PubMed] [Google Scholar]
- 3.Johansen JS, Christoffersen P, Møller S, Price PA, Henriksen JH, Garbarsch C, Bendtsen F. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 4.Johansen JS, Drivsholm L, Price PA, Christensen IJ. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer. 2004;46:333–340. doi: 10.1016/j.lungcan.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 6.Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12:3682–3694. doi: 10.3748/wjg.v12.i23.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70:163–170. [PubMed] [Google Scholar]
- 8.Zheng M, Cai WM, Zhao JK, Zhu SM, Liu RH. Determination of serum levels of YKL-40 and hyaluronic acid in patients with hepatic fibrosis due to schistosomiasis japonica and appraisal of their clinical value. Acta Trop. 2005;96:148–152. doi: 10.1016/j.actatropica.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 9.del Pilar Alatorre-Carranza M, Miranda-Díaz A, Yañez-Sánchez I, Pizano-Martínez O, Hermosillo-Sandoval JM, Vázquez-Del Mercado M, Hernández-Hoyos S, Martínez-Abundis R, Fafutis-Morris M, Segura-Ortega J, et al. Liver fibrosis secondary to bile duct injury: correlation of Smad7 with TGF-beta and extracellular matrix proteins. BMC Gastroenterol. 2009;9:81. doi: 10.1186/1471-230X-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Bartolí R, Planas R, Cabré E, Jimenez M, Urban A, Ojanguren I, Arnal J, Gassull MA. Bacterial translocation in cirrhotic rats. Its role in the development of spontaneous bacterial peritonitis. Gut. 1994;35:1648–1652. doi: 10.1136/gut.35.11.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 13.Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339–350. doi: 10.1055/s-2007-991511. [DOI] [PubMed] [Google Scholar]
- 14.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 15.Pinzani M, Knauss TC, Pierce GF, Hsieh P, Kenney W, Dubyak GR, Abboud HE. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol. 1991;260:C485–C491. doi: 10.1152/ajpcell.1991.260.3.C485. [DOI] [PubMed] [Google Scholar]
- 16.Johansen JS, Krabbe KS, Møller K, Pedersen BK. Circulating YKL-40 levels during human endotoxaemia. Clin Exp Immunol. 2005;140:343–348. doi: 10.1111/j.1365-2249.2005.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, Krusell L, Haus G, Utikal J, Schledzewski K, Scholtze J, et al. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–3228. doi: 10.1182/blood-2005-07-2843. [DOI] [PubMed] [Google Scholar]
- 18.Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- 19.Date M, Matsuzaki K, Matsushita M, Tahashi Y, Furukawa F, Inoue K. Modulation of transforming growth factor beta function in hepatocytes and hepatic stellate cells in rat liver injury. Gut. 2000;46:719–724. doi: 10.1136/gut.46.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J. 2002;365:119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]