Abstract
AIM: To explore the effects of siRNA silencing of PIK3CA on proliferation, migration and invasion of gastric cancer cells and to investigate the underlying mechanisms.
METHODS: The mutation of PIK3CA in exons 9 and 20 of gastric cancer cell lines HGC-27, SGC-7901, BGC-823, MGC-803 and MKN-45 was screened by polymerase chain reaction (PCR) followed by sequencing. BGC-823 cells harboring no mutations in either of the exons, and HGC-27 cells containing PIK3CA mutations were employed in the current study. siRNA targeting PIK3CA was chemically synthesized and was transfected into these two cell lines in vitro. mRNA and protein expression of PIK3CA were detected by real-time PCR and Western blotting, respectively. We also measured phosphorylation of a serine/threonine protein kinase (Akt) using Western blotting. The proliferation, migration and invasion of these cells were examined separately by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), wound healing and Transwell chambers assay.
RESULTS: The siRNA directed against PIK3CA effectively led to inhibition of both endogenous mRNA and protein expression of PIK3CA, and thus significantly down-regulated phosphorylation of Akt (P < 0.05). Furthermore, simultaneous silencing of PIK3CA resulted in an obvious reduction in tumor cell proliferation activity, migration and invasion potential (P < 0.01). Intriguing, mutant HGC-27 cells exhibited stronger invasion ability than that shown by wild-type BGC-823 cells. Knockdown of PIK3CA in mutant HGC-27 cells contributed to a reduction in cell invasion to a greater extent than in non-mutant BGC-823 cells.
CONCLUSION: siRNA mediated targeting of PIK3CA may specifically knockdown the expression of PIK3CA in gastric cancer cells, providing a potential implication for therapy of gastric cancer.
Keywords: Gastric cancer, Metastasis, PIK3CA, PI3K/Akt pathway, RNAi
INTRODUCTION
Gastric cancer is one of the most frequent cancers and is the second leading cause of cancer-related death worldwide[1-2]. Although diagnostic and surgical techniques as well as combined chemotherapy and radiotherapy for the treatment of gastric cancer have advanced in recent years, the overall 5-year survival rate is still less than 20%. Thus, it is necessary to further explore and investigate the tumorigenesis of gastric cancer and its novel therapy targets.
RNA interference (RNAi) refers to the inhibition of gene expression by small double-stranded RNA (dsRNA) molecules targeting specific mRNAs for degradation[3]. The discovery of RNAi has revolutionized our understanding of gene regulation, led to the development of new strategies for blocking gene function, and may yield RNA-based drugs to treat human disease[4]. To date, a great number of studies have demonstrated that RNAi-mediated gene silencing has promising therapeutic potential for cancer therapy[5].
PIK3CA encodes the key enzymatic subunit p110α of phosphatidylinositol 3-kinase (PI3K) and is located at 3q26.3[6]. Few studies have addressed PIK3CA expression in malignancies, although its mutation has been found in many human solid cancers, including breast, gastric and pituitary cancer[7-9] and it plays an essential role as an oncogene in tumor development and progression. Our previous studies have demonstrated that increased expression of PIK3CA in the cytoplasm of gastric cancer tissues was likely associated with lymph node metastasis[10]. However, the relationship between down-regulation of PIK3CA and proliferation as well as metastatic ability or invasion of gastric cancer cells and the mechanism underlying any such relationship remains largely unknown.
In this study, we investigated the effects of down-regulation of PIK3CA by small interfering RNA (siRNA) on proliferation, migration and invasion of two gastric cancer cell lines (BGC-823 and HGC-27) as well as p-Akt expression, with the aim of evaluating whether the expression of PIK3CA may be linked to tumor progression, and explored the underlying mechanisms.
MATERIALS AND METHODS
Detection of PIK3CA mutation in gastric cancer cell lines
Detection of PIK3CA mutation in exons 9 and 20 was performed in 5 gastric cancer cell lines (HGC-27, SGC-7901, BGC-823, MGC-803 and MKN-45), covering the majority of hot spots of PIK3CA gene mutation. The polymerase chain reaction (PCR) amplification primers were designed according to the study published by Lin et al[9]. PCR products were electrophoresed on 1.5% agarose gels to ensure their integrity before purification and DNA sequencing on an ABI 3730 XL DNA Analyzer (Applied Biosystems, Foster City, CA, United States) by Biosune Ltd (Shanghai, China).
siRNA synthesis and transfection
The siRNAs were designed and synthesized by GenePharma (Shanghai, China). PIK3CA siRNA: sense 5'-GGC UAA AGA AAG CCU UUA UTT-3', antisense 5'-AUA AAG GCU UUC UUU AGC CTT-3'; Negative control siRNA: sense 5'-UUC UCC GAA CGU GUC ACG UTT-3', antisense 5'-ACG UGA CAC GUU CGG AGA ATT-3'. All sequences were submitted to National Institutes of Health Blast program to ensure gene specificity. Human gastric carcinoma BGC-823 cells were conventionally cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen Inc., Carlsbad, CA, United States) supplemented with 2 mmol/L of L-Glutamine and 10% FBS at 5% CO2 and 37 °C. The cells were divided into 3 groups: control group (containing only transfection reagent), negative control group (transfected with negative control siRNA) and experimental group (transfected with PIK3CA-siRNA). When cells reached 80-90% confluency, siRNA transfections were conducted using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s recommendations. Total RNAs and proteins were prepared from samples collected before transfection and at 24 h and 48 h post transfection and used for real-time quantitative PCR or Western blotting analysis.
Real time quantitative PCR
Transcript abundance of PIK3CA and β-actin (internal control) was relatively quantified by quantitative real time PCR (qRT-PCR) on total RNA isolated from the three cell groups. Briefly, 1 μg of total RNA was reverse transcribed in a 25 μL reaction volume using oligo dT (15) primers and M-MLV reverse transcriptase (Promega'Madison, WI, United States). The PCR amplifications and fluorescence detections were carried out using the ABI Prism 7500 Sequence Detection System following the manufacturer′s instructions. For each sample, a relative quantity was calculated using the 2-ΔΔC(T) method[11]. Nucleotide sequences of specific primers for the selected genes were as follows: PIK3CA forward primer (5'-TGCTAAAGAGGAACACTGTCCA-3'), reverse primer: (5'-GGTACTGGCCAAAGATTCAAAG-3'); β-actin forward primer (5'-CTGAGCAGATCATGAAGAC-3'), reverse primer (5'-CTTGGTGGACGCATCCTGAG-3').
Western blotting
Cell lysates were prepared in a buffer containing 0.5 mmol/L Tris•HCl (pH 7.0), 0.1% beta-mercaptoethanol, 0.5 mmol/L ethylenediaminetetraacetic acid (pH 7.0), 0.5 mmol/L ethyleneglycol-bis (2-aminoethylether)-N,N,N',N'-tetraacetic acid (pH 7.0), 2 mmol/L leupeptin, 1 mmol/L phenylmethylsulfonyl fluoride, 2.5 mg/mL Aprotinin, 1 mmol/L dithiothreitol and 0.5% Triton X-100. After protein quantitation using the Branford assay, 30 μg of proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Life Sciences, Piscataway, NJ, United States). The membranes were blocked using phosphate buffered saline (PBS) (pH 7.4) containing 5% nonfat milk for 1 h, probed with primary antibody (anti-PIK3CA) (Cell Signaling Technology, Beverly, MA, United States) overnight at 4 °C. The membrane was then washed with PBST (PBS + 0.1% Tween-20) and incubated with a peroxidase-conjugated secondary antibody (goat anti-mouse IgG, Santa Cruz Biotechnology, Santa Cruz, CA, United States) for 1 h. Immunoreactive proteins were detected using an enhanced chemiluminescence detection reagent from BestBio (Shanghai, China). The membrane was stripped and reprobed with anti-phosphorylated Akt (Ser473), anti-Akt and anti-β-actin antibodies (Cell Signaling Technology).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The proliferation of BGC-823 and HGC-27 cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. This assay measures the dehydrogenase enzyme activity in metabolically active tumor cells, as reflected by the conversion of MTT to formazan, whose absorbance can be quantified by measuring at the wavelength of 570 nm. The production of formazan is proportional to the number of living cells, with the intensity of the produced color serving as an indicator of cell viability. Briefly, approximately 5 × 103 cells/well from the three groups were respectively seeded in a 96-well microtiter plate, each group had six parallel wells. At 24, 36 and 48 h post-transfection, 20 μL of MTT (Sigma Chemical Co, MO, United States) (5 g/L) labeling reagent was added to the designated wells and cells were incubated at 37 °C for 4 h and centrifuged at 1000 rpm for 5 min. The supernatant was removed, and 150 μL dimethyl sulfoxide (DMSO) (Sigma) was then added to each well. After shaking the plate for 15 min, the absorbance (A) at 570 nm was measured using Wellscan MK3 Automatic Microplate Reader. The blank control wells with medium only were set as zero absorbance. All experiments were performed at least three times.
Cell wound healing assay
To measure cell motility, 4 × 105 cells were seeded in 6-well plates. A central linear wound was created by scraping the cell monolayer with a 200 μL sterile pipette tip. The media were carefully changed to remove any floating cells and cultured at 5% CO2 and 37 °C. Migration of cells into the denuded areas in the scraped region was calculated at 24 h and 48 h, respectively. The wound at 0 h was considered 100% of the average gap.
Cell invasion assay
Cell invasion was assessed using Transwell chambers (Corning, NY, United States) with 50 μL sera-free DMEM containing 1 μg/μL Matrigel (BD, NJ, United States) in the upper chamber. Cells (4 × 104) were suspended with 200 μL DMEM without fetal bovine serum and placed onto the Matrigel. The lower chamber was filled with DMEM 500 μL containing 0.1 μg/μL fibronectin (Sigma). After 24 h incubation at 5% CO2 and 37 °C, the number of cells with Hematoxylin and Eosin (H and E) staining on the undersurface of the polycarbonate membranes (pore size 8 mm) was scored visually in 8 random fields using a light microscope.
Statistical analysis
Data were analyzed by GraphPad Prism 4.0 and SigmaPlot 8.0 software. The results were expressed as mean ± SD. Two or multiple comparisons were performed with Student's t-test or a one-way analysis of variance (ANOVA), respectively. A value of P < 0.05 was considered statistically significant.
RESULTS
Mutation analysis of PIK3CA
Among the 5 gastric cancer cell lines (HGC-27, SGC-7901, BGC-823, MGC-803 and MKN-45) analyzed, PIK3CA mutations in exon 9 or 20 were found in 2 of the 5 (40%) cell lines. HGC-27 cells harbored the G1633A (E545K) mutation in exon 9 and MKN-45 harbored the A3140G (H1047R) mutation in exon 20 (Table 1), which were consistent with a previous study in gastric cancer tissues[8].
Table 1.
PIK3CA mutation in gastric cancer cell lines
| Cell line | Nucleotide substitution | Amino acid change | Exon | Domain |
| HGC-27 | G1633A | E545K | 9 | Helical |
| MKN-45 | A3140G | H1047R | 20 | Kinase |
Detection of PIK3CA mutation in exons 9 and 20 was screened by PCR followed by sequencing in 5 gastric cancer cell lines (HGC-27, SGC-7901, BGC-823, MGC-803 and MKN-45). Only two cell lines carried mutations of PIK3CA. HGC-27 cells contained the G1633A (E545K) mutation in exon 9 and MKN-45 contained the A3140G (H1047R) mutation in exon 20.
To gain insight into the outcome through functional knockdown of PIK3CA, two gastric cancer cell lines (BGC-823 and HGC-27) harboring non-mutant and mutant PIK3CA, respectively, were selected to fulfill this task. The selection was based on the fact that in these three gastric cancer cell lines, the higher the expression of PIK3CA both at the mRNA and protein level, the more invasive the cells are[12].
RNAi decreases PIK3CA and p-Akt expression
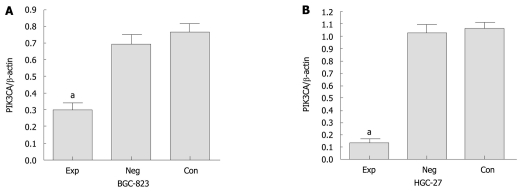
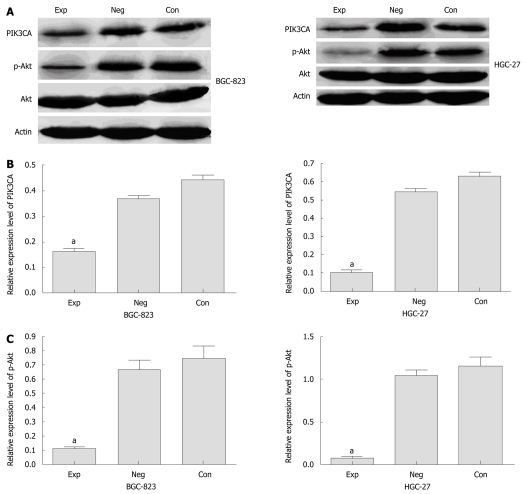
We analyzed the expression of PIK3CA mRNA in BGC-823 cells transfected with siRNA by qRT-PCR. Before transfection, PIK3CA mRNA was abundantly expressed among the three groups of cells, with no statistical significance between them (P < 0.05) (data not shown). However, PIK3CA mRNA expression was markedly decreased by about 70% in the experimental group compared with the two control groups (Figure 1). Similarly, densitometric analysis showed that PIK3CA protein expression in the experimental group was about 2.5- and 2-fold lower than those in the control group and negative control group, respectively (P < 0.05), while no statistical difference in PIK3CA protein expression was found between these two control groups (P > 0.05) (Figure 2A and B). Interestingly, the level of p-Akt protein in the experimental group was also dramatically down-regulated compared with the two control groups as shown in Figure 2A and C . Additionally, the levels of PIK3CA mRNA and protein were dramatically reduced by about 85% and 80% in the experimental HGC-27 cells in comparison with the two control groups (Figure 1, Figure 2A and B). A similar difference was also observed when the HGC-27 cells were assessed for protein expression of p-Akt (Figure 2A and C). Interestingly, no significantly statistical difference in PIK3CA protein levels was found in experimental wild-type BGC-823 and mutant HGC-27 cells (Figure 2B). These data indicated that siRNA silencing of PIK3CA led to obvious inhibition of mRNA and protein expression of PIK3CA in these two gastric cell lines, and decreased activation of Akt was probably due to constitutive inactivation of PIK3CA rather than changes in its protein levels.
Figure 1.
Expression analyses of PIK3CA mRNA determined by quantitative real time polymerase chain reaction at 24 h post-transfection. Values are shown as mean ± SD. Exp: Experimental group; Neg: Negative control group; Con: Control group. aP < 0.05 vs controls, n = 3.
Figure 2.
Protein expression of PIK3CA and p-Akt in gastric cancer cells. A: Expression analyses of PIK3CA and p-Akt protein in cells by Western blotting assay. The representative data are shown in triplicate experiments. β-actin: internal control protein; B and C: Statistical evaluation of relative expression of PIK3CA and p-Akt protein quantified by grey analyses with SigmaPlot 8.0 software. Exp: Experimental group; Neg: Negative control group; Con: Control group. aP < 0.05 vs controls, n = 3.
Effects of PIK3CA down-regulation on cell proliferation, migration and invasion
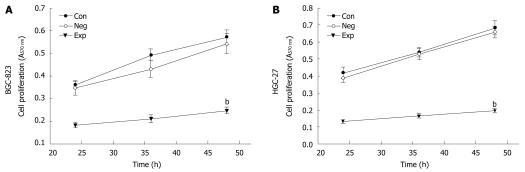
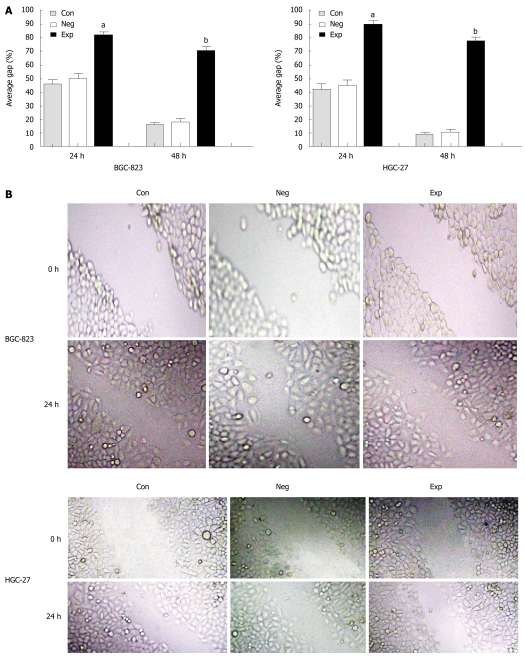
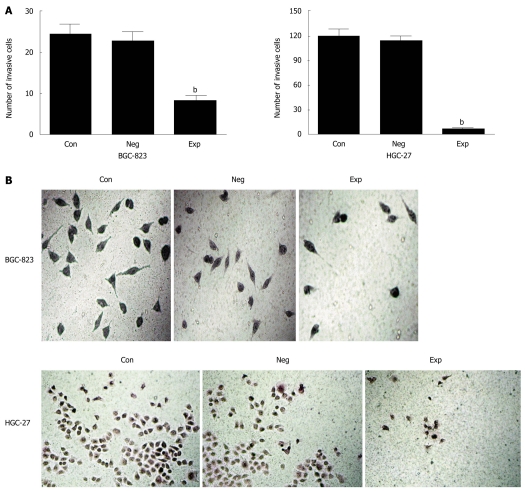
Because PIK3CA-siRNA was able to impair activation of the PI3K/Akt pathway, it was of significant interest and a priority to assess whether this siRNA had a functional effect on the biological properties of gastric cancer cells. To address this, we performed a MTT assay on gastric cancer cells transfected with siRNA against PIK3CA. As shown in Figure 3, transfection with PIK3CA-siRNA significantly decreased the proliferation of BGC-823 cells as compared with the controls (P < 0.01), while the proliferation of BGC-823 cells between the control group and the negative control group showed no statistical significance (P > 0.05), implying that knockdown of PIK3CA had an obvious inhibitory impact on the proliferation of BGC-823 cells. To examine the effect of PIK3CA-siRNA on cell motility, an in vitro wound-healing assay was performed. The results showed that the cells transfected with PIK3CA-siRNA had a reduced migration rate compared with the control groups at 24 h (P < 0.05) and 48 h (P < 0.01) (Figure 4). To further investigate the effect of PIK3CA-siRNA on cell invasion, we determined the invasion ability of the three groups of cells using the Transwell chambers assay. After incubation for 24 h, the number of control group and negative control group cells which had invaded the polycarbonate membrane of the Matrigel chamber was approximately 3.3- and 2.8-fold greater than that of the experimental group, respectively [(23.35 ± 1.37) and (20.24 ± 1.16) vs (6.98 ± 0.56)](P < 0.01) (Figure 5). The results of this experiment support the suggestion that PIK3CA-siRNA reduces invasion ability of gastric cancer BGC-823 cells. As expected, silencing of PIK3CA in mutant HGC-27 cells led to reduced cell proliferation and invasion to a greater extent than that in non-mutant BGC-823 cells (Figures 3, 4 and 5), implying that PIK3CA knockdown may preferentially inhibit proliferation and invasion of mutant PIK3CA cells.
Figure 3.
Cell proliferation assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Values are mean ± SD. Con: Control group; Neg: Negative control group; Exp: Experimental group. bP < 0.01 vs controls, n = 3.
Figure 4.
PIK3CA-siRNA reduced migration of BGC-823 and HGC-27 cells using an in vitro wound healing assay. A: The results were expressed as average gap and compared with the two control groups. The distance of the wound was measured at five reference points along the scratch wound. aP < 0.05 vs controls, bP < 0.01 vs controls, n = 3; B: A representative result of cell migration at 0 h and 24 h (original magnification, × 150). Con: Control group; Neg: Negative control group; Exp: Experimental group.
Figure 5.
Effect of PIK3CA silencing on tumor cell invasion. A: PIK3CA knockdown reduces invasion ability of gastric cancer cells; B: A representative result of cell invasiveness among the three groups of cells (original magnification of BGC-823 and HGC-27 cells, × 200, × 150, respectively). Con: Control group; Neg: Negative control group; Exp: Experimental group. bP < 0.01 vs controls, n = 3.
DISCUSSION
It is currently thought that gastric cancer develops through a complex process, such as the activation of oncogenes and/or the inactivation of tumor suppressor genes[13]. However, the critical underlying molecular mechanism of its progression is largely unclear. In recent years, many researchers have focused on signaling pathway deregulation in cancers. Among them, dysregulation of the PI3K/Akt pathway in a wide spectrum of human cancers has become a research hotspot.
The PI3Ks are heterodimers consisting of p110 catalytic and p85 regulatory subunits and have been linked to an extraordinarily diverse group of cellular functions, including differentiation, cell adhesion, apoptosis and tumor invasion[14]. Many of these functions relate to the ability of PI3K to activate its key downstream effector Akt[15,16]. Many studies have shown that Akt activity is detectable in a variety of tumors[17-19], including gastric cancer shown by our group[10]. Elevated phosphorylated Akt (p-Akt), the activated form, has been demonstrated in multiple malignancies[20] and is often functionally linked to tumor progression, such as in thyroid cancer[21], and metastasis, such as in gastric cancer[10]. As reported by Grille et al[16], Akt activation in cancer cells increased the motility required for tissue invasion and metastases and was consequently associated with poor prognosis in many cancers. Our previous study[12] also demonstrated that different gastric cancer cell lines (HGC-27, BGC-823 and SGC-7901) varied in their invasiveness which was associated with their expression level of PIK3CA.
In the present study, our results revealed that both PIK3CA mRNA and protein were markedly inhibited in two cell lines transfected with PIK3CA-siRNA, which is consistent with many studies showing that the introduction of a 21 nt dsRNA into cancer cells strongly suppressed the expression of specific mRNAs[22,23]. Furthermore, RNAi-directed targeting of PIK3CA in these cells could reduce the capability of cell proliferation, migration and invasion. More importantly, a low level of p-Akt in the experimental group was detected compared with the two control groups. The above evidence indicates that a robust knockdown of PIK3CA by siRNA may result in decreased catalytic activity of PI3K, subsequent de-phosphorylation of the downstream effector Akt, and thus low activity or aberrant inactivation of the PI3K/Akt pathway in these cells. Our data are in agreement with previous observations that PI3-kinase activity is solely caused by gene-dependent expression of the catalytic subunit p110α (PIK3CA)[24,25].
Interestingly, the Transwell chambers assay showed that PIK3CA mutant HGC-27 cells had an approximately 5-fold increased ability to invade the Matrigel (Figure 5) compared to PIK3CA non-mutant BGC-823 cells, suggesting that PIK3CA mutation contributed to cell invasion, which is consistent with a previous study in which PIK3CA mutations occur late in glioma progression[26].
Taken together, siRNA targeting PIK3CA effectively inhibits the proliferation and invasion of gastric cancer cells via aberrant inactivation of the PI3K/Akt pathway, and would be expected to become a new strategy for the therapy of gastric cancer regardless of PIK3CA mutation. However, like all other newly developed therapeutic methods, applying RNAi via siRNAs to living animals, especially humans, poses many challenges such as their poor stability and different effectiveness in different cell types[27,28]. Further studies will be required to develop efficient approaches for the delivery of siRNA into target cells.
ACKNOWLEDGMENTS
We thank Lu MY for kindly technical assistance and Qi YC for comments on the manuscript.
COMMENTS
Background
Gastric cancer is the second leading cause of cancer-related death worldwide, and no ideal approach is available to treat this disease. Thus, it is necessary to further explore and investigate the tumorigenesis of gastric cancer and its novel therapy targets. PIK3CA encoding the key enzymatic subunit p110α of phosphatidylinositol 3-kinase (PI3K), plays a vital role as an oncogene in tumor development and progression. Few studies have addressed PIK3CA expression in malignancies, although its mutation has been found in various human solid cancers. In addition, the relationship between PIK3CA expression and invasion of gastric cancer cells and the mechanism underlying any such relationship remains largely unknown.
Research frontiers
It is currently thought that gastric cancer develops through a complex process, such as the activation of oncogenes and/or the inactivation of tumor suppressor genes. However, the critical underlying molecular mechanism of its progression is largely unclear. In recent years, many researchers have focused on signaling pathway deregulation in cancers. Among them, dysregulation of the PI3K/Akt pathway in a wide spectrum of human cancers has become a research hotspot.
Innovations and breakthroughs
Previous studies have mainly focused on PIK3CA mutations in many human solid cancers. In the present study, the authors investigated the effects of the knockdown of PIK3CA by small interfering RNA on proliferation, migration and invasion of two gastric cancer cell lines with or without mutation of PIK3CA, with the aim of evaluating whether the expression of PIK3CA may be linked to tumor progression and may ultimately benefit from gastric cancer therapy regardless of the presence of PIK3CA mutations.
Applications
Functional knockdown of PIK3CA mediated by siRNA effectively inhibits the proliferation and invasion of gastric cancer cells with or without mutation of PIK3CA via aberrant inactivation of the PI3K/Akt pathway, and would be expected to become a new strategy for gastric cancer therapy. Further studies will be required to develop efficient approaches for the delivery of siRNA into target cells.
Terminology
PIK3CA gene, encoding the key catalytic subunit p110α of PI3K, is located on chromosome 3q26.3. AKT, a serine/threonine kinase, serving as the major downstream effector of PI3K, regulates many biological processes, such as proliferation, apoptosis and growth.
Peer review
The paper is well written and executed. The results are correctly described and commented.
Footnotes
Supported by Natural Science Foundation of Hunan Province, No. 09JJ3060; Health Bureau Fund of Guangzhou, No. 201102A213006; Education Bureau Fund of Guangzhou, No. 10A186
Peer reviewers: Tim Tak Kwok, Associate Professor, School of Biomedical Sciences, The Chinese University of Hong Kong, Shatin, NT, Hong Kong, China; Francesco Feo, Professor, Department of Biomedical Sciences, Section of Experimental Pathology and Oncology, University of Sassari, Via P, Manzella 4, 07100 Sassari, Italy; Hikaru Nagahara MD, PhD, Professor, Aoyama Hospital, Tokyo Women’s Medical University, 2-7-13 Kita-Aoyama, Minatoku, Tokyo, 107-0061, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Milosavljevic T, Kostic-Milosavljevic M, Jovanovic I, Krstic M. Gastrointestinal and liver tumours and public health in Europe. Eur Rev Med Pharmacol Sci. 2010;14:259–262. [PubMed] [Google Scholar]
- 3.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 4.Zamore PD. RNA interference: listening to the sound of silence. Nat Struct Biol. 2001;8:746–750. doi: 10.1038/nsb0901-746. [DOI] [PubMed] [Google Scholar]
- 5.Wilda M, Fuchs U, Wössmann W, Borkhardt A. Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi) Oncogene. 2002;21:5716–5724. doi: 10.1038/sj.onc.1205653. [DOI] [PubMed] [Google Scholar]
- 6.Volinia S, Hiles I, Ormondroyd E, Nizetic D, Antonacci R, Rocchi M, Waterfield MD. Molecular cloning, cDNA sequence, and chromosomal localization of the human phosphatidylinositol 3-kinase p110 alpha (PIK3CA) gene. Genomics. 1994;24:472–477. doi: 10.1006/geno.1994.1655. [DOI] [PubMed] [Google Scholar]
- 7.Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 8.Li VS, Wong CW, Chan TL, Chan AS, Zhao W, Chu KM, So S, Chen X, Yuen ST, Leung SY. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5:29. doi: 10.1186/1471-2407-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y, Jiang X, Shen Y, Li M, Ma H, Xing M, Lu Y. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr Relat Cancer. 2009;16:301–310. doi: 10.1677/ERC-08-0167. [DOI] [PubMed] [Google Scholar]
- 10.Liu JF, Zhou XK, Chen JH, Yi G, Chen HG, Ba MC, Lin SQ, Qi YC. Up-regulation of PIK3CA promotes metastasis in gastric carcinoma. World J Gastroenterol. 2010;16:4986–4991. doi: 10.3748/wjg.v16.i39.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T) ) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Liu JF, Li W, Qi YC. Effects of PIK3CA overexpression on invasion of gastric cancer cells. Chin J Cancer Prev Treat. 2010;17:1727–1729. [Google Scholar]
- 13.Endoh Y, Sakata K, Tamura G, Ohmura K, Ajioka Y, Watanabe H, Motoyama T. Cellular phenotypes of differentiated-type adenocarcinomas and precancerous lesions of the stomach are dependent on the genetic pathways. J Pathol. 2000;191:257–263. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH631>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 15.Takeda A, Osaki M, Adachi K, Honjo S, Ito H. Role of the phosphatidylinositol 3’-kinase-Akt signal pathway in the proliferation of human pancreatic ductal carcinoma cell lines. Pancreas. 2004;28:353–358. doi: 10.1097/00006676-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- 17.Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- 18.Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Tanno S, Skele KL, Hoffman JP, Testa JR. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem. 2002;87:470–476. doi: 10.1002/jcb.10287. [DOI] [PubMed] [Google Scholar]
- 19.Semba S, Moriya T, Kimura W, Yamakawa M. Phosphorylated Akt/PKB controls cell growth and apoptosis in intraductal papillary-mucinous tumor and invasive ductal adenocarcinoma of the pancreas. Pancreas. 2003;26:250–257. doi: 10.1097/00006676-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Cicenas J. The potential role of Akt phosphorylation in human cancers. Int J Biol Markers. 2008;23:1–9. doi: 10.1177/172460080802300101. [DOI] [PubMed] [Google Scholar]
- 21.Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004;41:161–170. doi: 10.1136/jmg.2003.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerutti H. RNA interference: traveling in the cell and gaining functions? Trends Genet. 2003;19:39–46. doi: 10.1016/s0168-9525(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 23.Dykxhoorn DM, Novina CD, Sharp PA. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2003;4:457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 24.Singh B, Reddy PG, Goberdhan A, Walsh C, Dao S, Ngai I, Chou TC, O-Charoenrat P, Levine AJ, Rao PH, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16:984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 26.Broderick DK, Di C, Parrett TJ, Samuels YR, Cummins JM, McLendon RE, Fults DW, Velculescu VE, Bigner DD, Yan H. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004;64:5048–5050. doi: 10.1158/0008-5472.CAN-04-1170. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen T, Menocal EM, Harborth J, Fruehauf JH. RNAi therapeutics: an update on delivery. Curr Opin Mol Ther. 2008;10:158–167. [PubMed] [Google Scholar]
- 28.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]