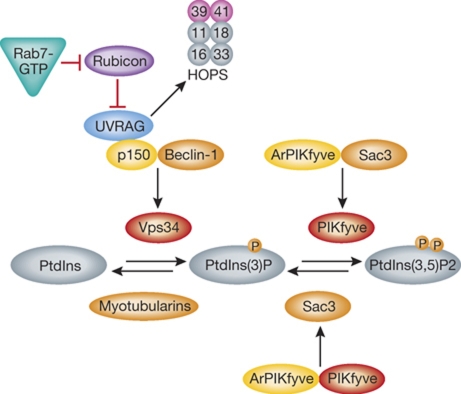
Figure 5.
Phosphatidylinositide regulation on endosomes. On EEs, PtdIns(3)P is synthesized by the kinase VPS34, which forms a core complex together with p150 and Beclin-1. The complex binds on endosomes to UVRAG (Itakura et al, 2008), which is normally inhibited by a Rab7 effector, Rubicon (Sun et al, 2010). Once activated, Rab7 sequesters Rubicon from UVRAG, allowing it to activate the HOPS complex. Dephosphorylation of PtdIns(3)P is catalyzed by members of the Myotubularin family. The kinase responsible for conversion of PtdIns(3)P to PtdIns(3,5)P(2) is PIKfyve (Fab1p) (Gary et al, 1998; Ikonomov et al, 2002). It forms an active complex with its activator ArPIKfyve (Vac14p) and the phosphatase Sac3 (Fig4p). This complex is required for both the kinase and the phosphatase activities (Sbrissa et al, 2008; Ikonomov et al, 2009).