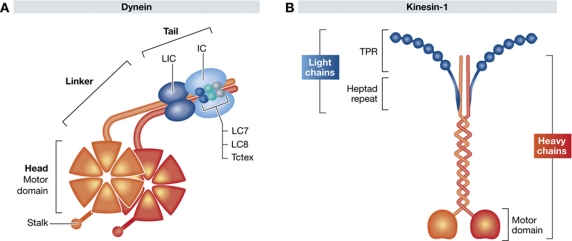
Figure 1.
Schematic representation of the subunit composition of dynein and kinesin-1. (A) The motor containing cytoplasmic dynein heavy chains are shown in orange and associated intermediate and light chains in shades of blue. The motor domain is composed of six AAA ATPase domains arranged in a hexameric ring from which a microtubule binding stalk projects. The N-terminal tail of the heavy chain mediates its dimerization and contains the binding sites for two intermediate chains (ICs) and two light intermediate chains (LICs). The two intermediate chains (ICs) also interact with three pairs of light chains: Tctex, LC7 and LC8. (B) Kinesin-1 is a heterotetramer composed of two motor containing heavy chains (orange) and two light chains (blue). The microtubule binding motor domain is found in the N-terminus of the heavy chain. The light chains associate with the heavy chains via heptad repeat regions in their N-terminus. The C-terminal half of the light chains is composed of six tetratricopeptide repeats (TPR), which represent cargo binding domains.