Abstract
Wider use of pharmacological models would facilitate the development of new drugs for Alzheimer's disease (AD), The two main models currently used are based on the cholinergic and glutamatergic hypotheses of AD, Although they lead to some of the attention and memory impairment observed in AD, they do not fully reproduce the AD pattern. The few studies that used a combination modeling approach, ie, the simultaneous administration of several drugs with the aim of impairing several neurotransmitters or different aspects of a single system, have reported no or marginal cumulative effect. On the basis of current understanding of glutamate and acetylcholine involvement in AD pathophysiology, we suggest that models using selective muscarinic-1 (M1) receptor blockers would better mimic the status of the cholinergic system in AD, This kind of model might be suitable for the assessment of drugs that do not act directly on the cholinergic system.
Keywords: Alzheimer's disease, dementia, model, scopolamine, ketamine, pirpirenzepine, acetylcholine, glutamate
Abstract
Un empleo más generalizado de modelos farmacológicos facilitaría el desarrollo de nuevos fármacos para la enfermedad de Alzheimer (EA). Los dos modelos principales actualmenie en uso se basan en las hipótesis colinérgica y glutamatérgica de la EA. Aunque ambos modelos se orientan hacia algunos de los deterioros en la atención y en la memoria observados en la EA, ellos no reproducen totalmente el patrón de la EA. Los pocos estudios que utilizan una combination de modelos (ej. la administration simultánea de varios fármacos con el objetivo de afectar algunos neuroiransmisores o diferentes aspectos de un sistema único) no han informado de ningún efecto significativo, a lo más el efecto acumulativo es marginal. De acuerdo con el conocimiento actual del compromiso del glutamato y de la acetílcolina en la fisiopatología de la EA, nosotros sugerimos que los modelos que emplean bloqueadores selectivos de los receptores muscarinicos-1 (M1) podrían imitar mejor la situation del sistema colinérgico en la EA, Esta clase de modelo sería conveniente para la evaluación de fármacos que no actúan directamente en el sistema colinérgico.
Abstract
Le recours plus fréquent aux modèles pharmacologiques devrait faciliter le développement de nouvelles thérapeutiques dans la maladie d'Alzheimer (MA). Les deux principaux modèles utilisés actuellement sont fondés sur l'hypothèse d'une implication du système cholinergique et du système glutamatergique dans la maladie d'Alzheimer. Mais si l'on peut observer dans ces modèles l'apparition de certains des troubles de l'attention et de la mémoire observés au cours de la MA, ils ne génèrent pas la totalité des manifestations de la pathologie. Dans les quelques études qui ont utilisé un modèle combinant l'administration simultanée de plusieurs molécules dans le but d'altérer différents neurotransmetteurs ou différentes étapes d'un même système, l'effet cumulatif obtenu a été nul ou négligeable. En nous appuyant sur nos connaissances actuelles de l'implication du glutamate et de l'acetylcholine dans la physiopathologie de la MA, nous proposons d'utiliser des inhibiteurs sélectifs des récepteurs muscariniques-1 (M1) pour reproduire au mieux l'état du système cholinergique dans la MA, Ce type de modèle pourrait permettre d'évaluer des molécules qui n'agissent pas directement sur le système cholinergique.
All the professionals involved are convinced that finding effective treatments for Alzheimer's disease (AD) should be a priority for the pharmaceutical industry. AD is a wonderful challenge for industry. However, research and development in this field can also be a risky business. There is currently no consensus on the pathophysiology of AD on which drug development can rely. The clinicopathologic picture that we call AD may actually be a syndrome, with many possible causes. As a consequence, we still have no reliable, positive diagnostic test that can be applied on an individual basis, which leads to the risk of recruiting very heterogeneous patient populations for clinical trials. The low response rate to acetylcholine esterase inhibitors probably illustrates these uncertainties.
Before starting expensive trials, pharmaceutical companies clearly need to assess the validity of the underlying concept in the early phases of development. Part of the answer can come from animal models. However, if a pharmacological effect is observed, it must still be confirmed in humans.
Some advocate early administration to patients, but this is not necessarily the simplest method. The risk of heterogeneous recruitment to clinical trials is an important point. If the goal is to measure clinical improvement, the drug will probably be administered for a long period of time. If the trial intends to assess changes in surrogate markers, these must be defined. Recruiting groups homogeneous for a selected marker can be difficult and time-consuming, and at this phase of development we need to go as fast as possible. Keeping pools of untreated patients at hand for this purpose, and depriving them of currently available drugs, is ethically questionable.
It is easier and faster to work with healthy volunteers, and, better, young healthy volunteers. This requires the use of models, in which the putative drug is evaluated for its ability to reverse either induced cognitive impairment or associated markers (using electroencephalogram [EEG], positron emission tomography [PET] scan, and functional magnetic resonance imaging [fMRI] changes), or both.
The scopolamine model
Scopolamine is a nonselective,1 competitive2 muscarinic receptor blocker. The scopolamine model has its roots in the cholinergic hypothesis of aging and AD, and has played a major role in its construction, which we will recall briefly here.
From the beginning of the 20th century until the midfifties, scopolamine was used in obstetrics to induce a twilight state and amnesia during childbirth.3 In the sixties and seventies, it became obvious that regions rich in cholinergic afférents, such as the hippocampus, were involved in memory processes (see reference 4 for a review). In 1965, acetylcholine esterase activity was shown to be lowered in AD.5 In 1974, Drachman and Leavitt6 administered scopolamine to healthy young volunteers, who then displayed a memory profile very close to that observed in elderly people.
Two to three years later, three independent research teams7-9 reported a decreased activity of choline acetyltransferase (CAT), the enzyme responsible for acetylcholine (Ach) synthesis, in the cortex of AD patients. This decrease was shown to be correlated with brain lesions and clinical status.10-11 It was soon found that neuronal loss occurs in the forebrain basal nucleus of Meynert12 and medial septal nucleus,13 which are the source of neocortical and hippocampal cholinergic afferent fibers, respectively.14-16 In its early version,4 the cholinergic hypothesis stated nothing about etiological factors, did not address the additional roles that ACh dysfunction may play in other neurobehavioral disturbances of aging and dementia, and did not imply any exclusive or solitary involvement of the cholinergic system in age-related memory loss. It was a kind of “black box” model, in which an unknown pathophysiological process induces deficiency in various neurotransmission pathways thought to be responsible for the cognitive and behavioral aspects of aging and dementia. Despite obvious shortcomings (see references 17-20 for review and discussion), the cholinergic hypothesis legitimized the development of the cholinergic drugs we prescribe today and the administration of scopolamine as a model of investigation for AD research.
The scopolamine model was used in cognitive research to study the clinical correlates of ACh deficiency (see reference 21 for a review). It was applied to elderly subjects and AD patients22-33 as a marker of cholinergic sensitivity, with the purpose of improving the diagnosis and staging of the disease. It failed, however, to predict cognitive decline on the basis of the subjects' sensitivity.34
Animal studies assessing the reversal of scopolamineinduced memory impairment by various compounds are too numerous to be cited exhaustively. This approach has also been used in humans with the following molecules: physostigmine,35-40 velnacrine,40 choline,41 RO 15-1788,39 moclobemide,42,43 RU 41656,44 L-α-glycerylphosphoryleholine,45 oxiracetam,46 aniracetam and piracetam,47 tenilsetam,48 BMY 21502,49 D-cycloserine,50 SDZ ENS-163,51 and ZK-93426.52 However, the scopolamine model has not become a standard tool in the early assessment of drugs.
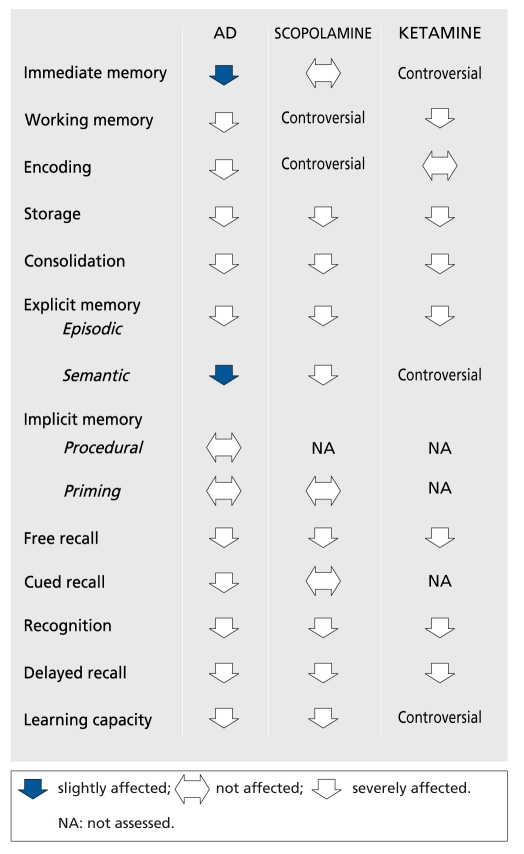
One reason for this is that the cognitive changes induced by scopolamine do not really mimic the AD picture. The details of the differences listed in Figure 1 (based on references 28, 40, and 53-63) are open to discussion, but there is a general agreement on the fact that, as Wesnes40 wrote, all the scopolamine-induced deficiencies are also observed in AD, while the reverse is not always true. The same is observed in neurological investigations. The electrophysiological effects of scopolamine (reviewed in reference 64) are close on EEG and similar on visual evoked potentials to those of AD. In PET65-68 and single photon emission computed tomography (SPECT)69 studies, scopolamine induces cerebral blood flow (CBF) and glucose metabolism changes, which are sometimes divergent and region-specific, but in all cases different from the pattern observed in AD.
Figure 1. Memory dysfuction in Alzheimer's disease (AD) and after scopolamine or ketamine.
The ketamine model
Ketamine is a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist.70-71 Its administration in order to produce a model is the correlate of the glutamatergic hypothesis of AD (reviewed in reference 72). Two, apparently opposite, glutamatergic hypotheses have been proposed. The excitotoxic hypothesis states that there is a glutamatergic hyperactivity in AD. Domoic acid poisoning in humans was responsible for irreversible memory loss.73 Neuronal74 and astroglial75 glutamate transporter dysfunction in AD could result in excess glutamate in the synaptic cleft and in excitotoxic neuronal damage. This hypothesis is consistent with the beneficial effects of memantine76 and lamotrigine77 in AD patients. Some findings provide a link with the histopathological lesions that are the hallmarks of AD. Kainic acid injection in the rat leads to decreased neuronal amyloid precursor protein (APP) 695 mRNA owing to neuronal death, and increased astrocytic APP 770 mRNA,78 which has been found selectively increased in AD in comparison with other neurodegenerative disorders79 and associated with plaques.80 β- Amyloid sensitizes neurons to glutamate toxicity81 and also enhances glutamate release by macrophages.82 Furthermore, in neuronal culture, glutamate was shown to enhance tau gene expression83 and induce paired helical filaments similar to those found in AD.84 The hypoglutamatergic hypothesis has been extensively reviewed elsewhere (see Newcomer et al, in this issue). The ketamine model is its application. Ketamine is mainly used in the field of schizophrenia research to provoke psychotomimetic as well as cognitive effects.85-94 These studies did not all assess the same functions or use the same paradigm to assess a particular function. Despite this limitation, when these studies are summarized and the profile of ketamine effects compared with that of AD (Figure1),60-63 the situation is the same as that for the scopolamine model: the functions affected by ketamine are affected in AD, but the reverse is not necessarily the case.
Future directions
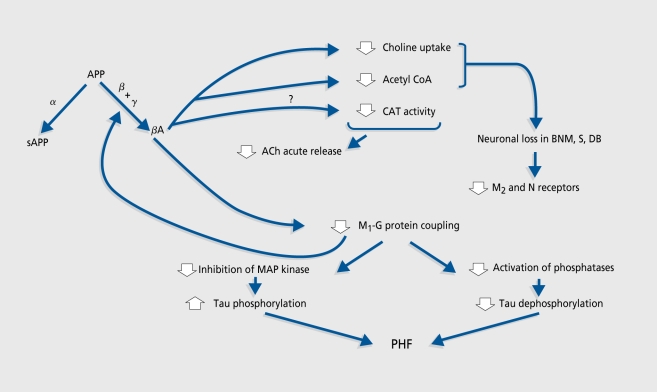
The two main models proposed thus lead to some of the attentional and memory impairment observed in AD, but do not fully reproduce the AD pattern. Two options are therefore possible. Since multiple neurotransmitter systems are affected in AD, it has been suggested95 that combination modeling through simultaneous administration of drugs that impair several neurotransmitters or different aspects of a single system could mimic the AD pattern more closely. The fewpublished studies on this strategy add mecamylamine,96 m-chlorophenylpiperazine (mCPP),97 metergoline, or haloperidol98 to scopolamine, and report no98 or marginal96-97 cumulative effect. Although NMDA antagonists were shown to potentiate the amnesic effect of scopolamine in the rat,58 no study on this combination in humans has been published to date. Beyond the weakness of their effects, combined models are so complex that they become difficult to understand - and particularly difficult to manipulate - in the assessment of cognitive enhancers. Another method is to take advantage of the recent advances in our understanding of AD. Currently available data support the view that neuronal and synaptic loss, rather than secondary neurotransmission disruption, is most likely responsible for cognitive changes in AD.99 They also allow attempts to integrate neurotransmitter changes into a more comprehensive theoretical framework. The cholinergic hypothesis in its current version (Figure 2) focuses on the reciprocal modulatory influences of cholinergic transmission and APP processing (reviewed in references 100 and 101). β-Amyloid (βA) is known to be neurotoxic at high (micromolar) concentrations.102 In vitro, soluble βA at picomolar to nanomolar levels is not toxic but does inhibit synthesis and stimulated release of ACh.103-105 Studies on the possible inhibitory effect of βA on CAT activity104-106 gave divergent results. βA appears to exert its effect on ACh synthesis and release through depletion of ACh precursors. It has been shown to disrupt the activity of pyruvate dehydrogenase,106 which generates acetyl coenzyme A (CoA) from pyruvate and was found to be decreased in the cortex of AD patients,107 and to inhibit highaffinity choline uptake.104 This could have an indirect neurotoxic effect, since cholinergic neurons deprived of choline have been shown to break down phosphatidylcholine from intracellular organelle membranes to provide additional choline.108
Figure 2. Amyloid precursor protein (APP) is processed either by β-secretase into a nonamyloidogenic pathway or by β- and γ-secretases to produce β-amyloid peptide (βA). βA could decrease choline acetyltransferase (CAT, the acetylcholine synthesis enzyme) activity. It lowers the availability of the substrates for acetylcholine (ACh) synthesis by impairing high-affinity choline uptake and acetyl coenzyme A (acetyl CoA) production; therefore ACh release is also diminished. Choline deprivation could initiate the so-called “autocannibalism” process through which ACh neurons break down membrane phosphatidylcholine to increase choline availability. Autocannibalism could be partly responsible for neuronal loss in the basal nucleus of Meynert (BNM), medial septal nucleus (S), and nucleus of the diagonal band of Broca (DB), and for the observed decrease in muscarinic M2 and nicotinic (N) receptor densities, which are mainly presynaptic. Muscarinic M, receptors are mainly postsynaptic and their density is not affected in Alzheimer's disease. However, they are probably dysfunctional because of receptor-G protein uncoupling, with two consequences: (i) lowered M, signal transduction favors the amyloidogenic APP processing pathway, which further aggravates uncoupling; and (ii) through loss of inhibition of mitogen-activated protein (MAP) kinase, which results in increased tau protein phosphorylation, and inhibition of phosphatase, which results in a lesser dephosporylation of tau, it favors the formation of paired helical filaments (PHF).
Although there is no general consensus (see reference 109 for review), it is thought that postsynaptic muscarinic Mi acetylcholine receptor (AChR) density is unchanged in AD, while those of presynaptic M2 and nicotinic AChRs are reduced.110,111 It has been shown that activation of protein kinase C through Mj (and M3) AChRs lowers βA production by favoring the nonamyloidogenic processing of APP112,113 Despite their unchanged density, M1 receptors could be dysfunctional114,115 because of defective coupling to Gq/11 proteins.116 This could lead to increased βA production, which would further impair M1 AChR signal transduction.117 M1 AchR-G protein uncoupling could also favor protein tau phosphorylation and thus paired helical filament formation through disinhibition of mitogen-activated protein (MAP) kinases118 and decreased efficiency of taudephosphorylation.119
Despite the uncertainties that remain, it is clear that the cholinergic deficiency can no longer be seen as a late consequence of neuropathological changes, but at least as a contributor to the cascade of events leading to fullblown dementia.
The glutamatergic hypothesis has also been revisited. Its current version (see Newcomer et al, in this issue) reconciles the former hyper- and hypoglutamatergic hypotheses by proposing a two-stage process.
In the first stage, βA increases the sensitivity of NMDA receptors to normal concentrations of glutamate, leading to destruction of NMDA-bearing GABAergic (GABA: gamma-aminobutyric acid), noradrenergic (NE), and serotonergic (5-HT) neurons, which have an inhibitory action on basal forebrain cholinergic, anterior thalamic glutamatergic, and cortical neuropeptide Y (NPY) neurons either directly (GABA, NE) or through activation of GABA neurons (5-HT).
This loss of inhibition leads to hyperstimulation of cortical and corticolimbic neurons, which then degenerate, as do the hyperactive cholinergic, glutamatergic, and NPY neurons.
This suggests that for both cholinergic and glutamatergic neurons, the hypoactive, symptomatic stage is preceded by a hyperactive one. These new versions of the cholinergic and glutamatergic hypotheses make it necessary for us to reappraise our models. The goal of an acute pharmacological model is to transiently reproduce the hypoactive, symptomatic stage. According to the scheme proposed by Newcomer et al elsewhere in this issue, NMDA blockers induce transient hyperactivity of basal forebrain cholinergic, anterior thalamic glutamatergic, and cortical NPY neurons. It is likely that the mechanism by which acute administration of NMDA blockers produces memory impairment is different and does not involve the two-stage sequence proposed as a chronic model. The finding that pretreatment with haloperidol reduces ketamineinduced impairment in executive cognitive functions91 nonetheless suggests that the cognitive effect of NMDA blockade is indirect and nonselective. Higher selectivity, which would also avoid psychotomimetic symptomatology, might be achieved by acting downstream of the NMDA receptor. For the particular posterior cingulate and retrosplenial region, the best choice would be to give m3 and/or kainate receptor blockers. Another target of choice is the hippocampus, in which the most common muscarinic receptor is the mi subtype; the m2 subtype represents 15% and the m3 subtype globally less than 12%.120 Moreover, specific blockade of the m1 receptors would best reproduce their status in AD, where they are hypostimulatcd (because of presynaptic neuronal loss) and dysfunctional. The only molecule which is more or less selective for the mi receptor121 and available for human use is pirenzepine. It is said to cross the blood-brain barrier poorly,122 but very few studies have assessed its central effects in man123-125 and we think it deserves further study.
Do neurotransmitter-based pharmacological models have a future?
The way the cognitive symptoms are produced in AD is complex and many therapeutic strategies already in development address βA metabolism and toxicity,126-128 rather than cholinergic deficiency. However, D-cycloserine, which does not act on the cholinergic system but modulates the NMDA receptor, has been shown to attenuate the effect of scopolamine on memory.50 Moclobemide, a selective monoamine oxidase A (MAOA) inhibitor,42,43 and thyrotropin-releasing hormonc (TRH)129 were also able to partly reverse the scopolamine-induced deficits. In the animal, the same has been observed with estrogens130 and GM1 gangliosides.131 Given these data and the current view that we have on the involvement of the cholinergic deficiency, it is very possible that new compounds, which do not act directly on the cholinergic system, could be effective on cholinergic models. Neurotransmitter-based models still have a place in our armamentarium, although efforts should be made to develop other approaches.
Conclusion
Whatever the model chosen, we must admit that it is e impossible to reproduce the full AD cognitive pattern. Instead, we can try to produce some aspects of memory it impairment, which is considered as the core symptom of:- the disease. The method used should be as simple and selective as possible in order to allow its manipulation. Selective ligands are currently in development132; accelerating the toxicological studies of these compounds could allow us to work this way in the near future.
Selected abbreviations and ancronyms
- Ach
acetylcholine
- AChR
acetylcholine receptor
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- βA
β-amyloid
- CAT
choline acetyltransferase
- NMDA
N-methyl-D-aspartate
- NPY
neuropeptide Y
Contributor Information
Christian Gilles, CNS Aging Research, FORENAP - Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Stéphane Ertlé, CNS Aging Research, FORENAP - Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
REFERENCES
- 1.Bolden C., Cusack B., Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. . J Pharmacol Exp Ther. 1992:576–580. [PubMed] [Google Scholar]
- 2.Yamamura HI., Snyder SH. Muscarinic cholinergic receptor binding in the longitudinal muscle of the guinea pig ileum with [3H]quinuclidinyl benzilate. . Mol Pharmacol. 1974;10:861–867. [Google Scholar]
- 3.Gauss CJ. Geburten in kunstlichem Dâmmerschlaf. . Arch Gynaekol. 1906;78:579–631. [Google Scholar]
- 4.Bartus RT., Dean RL., Pontecorvo MJ., Flicker C. The cholinergic hypothesis: a historical overview, current perspective, and future directions. Ann N Y Acad Sci. 1985;444:332–358. doi: 10.1111/j.1749-6632.1985.tb37600.x. [DOI] [PubMed] [Google Scholar]
- 5.Pope A., Hess HH., Lewin E. Microchemical pathology of the cerebral cortex in pre-senile dementias. . Trans Am Neurol Assoc. 1965;89:15–16. [PubMed] [Google Scholar]
- 6.Drachman DA., Leavitt J. Human memory and the cholinergic system: a relationship to aging? . Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 7.Bowen DM., Smith CB., White P., Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. . Brain. 1976;99:459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 8.Davies P., Maloney AJF. Selective loss of central cholinergic neurons in Alzheimer's disease. . Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 9.Perry EK., Perry RH., Blessed G., Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. . Lancet. 1977;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- 10.Perry EK., Tomlinson BE., Blessed G., Bergmann K., Gibson PH., Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. . BMJ. 1978;2:1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mountjoy CQ., Rossor MN., Iversen LL., Roth M. Correlation of cortical cholinergic and GABA deficits with quantitative neuropathological findings in senile dementia. . Brain. 1984;107:507–518. doi: 10.1093/brain/107.2.507. [DOI] [PubMed] [Google Scholar]
- 12.Whitehouse PJ., Price DL., Clark AW., Coyle JT., DeLong MR. Alzheimer's disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. . Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 13.Nakano I., Hirano A. Loss of large neurons in the medial septal nucleus in an autopsy case of Alzheimer's disease. . J Neuropathol Exp Neurol. 1982;41:341. [Google Scholar]
- 14.Mesulam MM., Mufson EJ., Levey AI., Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. . J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 15.Everitt BJ., Sirkià TE., Roberts AC., Jones GH., Robbins TW. Distribution and some projections of cholinergic neurons in the brain of the common marmoset, . Callithrix jacchus. J Comp Neurol. 1988;271:533–558. doi: 10.1002/cne.902710406. [DOI] [PubMed] [Google Scholar]
- 16.Alonso JR., U HS., Amaral DG. Cholinergic innervation of the primate hippocampal formation. II. Effects of fimbria/fornix transection. . J Comp Neurol. 1996;375:527–551. doi: 10.1002/(SICI)1096-9861(19961125)375:4<527::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Bartus RT., Dean RL., Fisher SK. Cholinergic treatment for age-related memory disturbances: dead or barely coming of age? In: Crook, T, Bartus R, Ferris S, Gershon S, eds. . Treatment Development Strategies for Alzheimer's Disease. Madison, Wis: Mark Powley Associates. 1986:421–450. [Google Scholar]
- 18.Hagan JJ. The status of the cholinergic hypothesis of dementia. In: Nicholson D, ed. . Anti-dementia Agents. Research and Prospects for Therapy. London, UK: Academic Press. 1994:85–138. [Google Scholar]
- 19.Salter M., Bruno JP., Himmelheber AM. Cortical acetylcholine and attention: neuropharmacological and cognitive principles directing treatment strategies for cognitive disorders. In: Brioni JD, Decker MW, eds. . Pharmacological Treatments of Alzheimer's Disease: Molecular and Neurobiological Foundations. New York, NY: Wiley-Liss. 1997:105–128. [Google Scholar]
- 20.Francis PT., Palmer AM., Snape M., Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. . J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lydon RG. Cholinergic neurons and memory: an historical perspective and overview of current research. In: Stone TW, ed. CMS . Neurotransmitters and Neuromodulators: Acetylcholine. London, UK: CRC Press. 1995:197–232. [Google Scholar]
- 22.Richardson JS., Miller PS., Lemay JS., et al. Mental dysfunction and the blockade of muscarinic receptors in the brain of the normal elderly. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:651–654. doi: 10.1016/0278-5846(85)90034-x. [DOI] [PubMed] [Google Scholar]
- 23.Sunderland T., Tariot P., Murphy DL., Weingartner H., Mueller EA., Cohen RM. Scopolamine challenges in Alzheimer's disease. . Psychopharmacology. 1985;87:247–249. doi: 10.1007/BF00431817. [DOI] [PubMed] [Google Scholar]
- 24.Sunderland T., Tariot PN., Mueller EA., Murphy DL., Weingartner H., Cohen RM. Cognitive and behavioral sensitivity to scopolamine in Alzheimer patients and controls. . Psychopharmacol Bull. 1985;21:676–679. [PubMed] [Google Scholar]
- 25.Sunderland T., Tariot PN., Cohen RM., Weingartner H., Mueller EA., Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose-response study. . Arch Gen Psychiatry. 1987;44:418–426. doi: 10.1001/archpsyc.1987.01800170032006. [DOI] [PubMed] [Google Scholar]
- 26.Sunderland T., Tariot PN., Newhouse PA. Differential responsivity of mood, behavior, and cognition to cholinergic agents in elderly neuropsychiatrie populations. . Brain Res. 1988;472:371–389. doi: 10.1016/0006-8993(88)91227-9. [DOI] [PubMed] [Google Scholar]
- 27.Zemishlany Z., Thome AE. Anticholinergic challenge and cognitive functions: a comparison between young and elderly normal subjects. . IsrJ Psychiatry Relat Sci. 1991;28:32–41. [PubMed] [Google Scholar]
- 28.Flicker C., Ferris SH., Serby M. Hypersensitivity to scopolamine in the elderly. . Psychopharmacology. 1992;107:437–441. doi: 10.1007/BF02245172. [DOI] [PubMed] [Google Scholar]
- 29.Gitelman DR., Prohovnik I. Muscarinic and nicotinic contributions to cognitive function and cortical blood flow. . Neurobiol Aging. 1992;13:313–318. doi: 10.1016/0197-4580(92)90044-x. [DOI] [PubMed] [Google Scholar]
- 30.Ray PG., Meador KJ., loring DW., Zamrini EW., Yang XH., Buccafusco JJ. Central cholinergic hypersensitivity in aging. . J Geriatr Psychiatry Neurol. 1992;5:72–77. doi: 10.1177/002383099200500203. [DOI] [PubMed] [Google Scholar]
- 31.Molchan SE., Martinez RA., Hill JL., et al. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. . Brain Res Brain Res Rev. 1992;17:215–226. doi: 10.1016/0165-0173(92)90017-g. [DOI] [PubMed] [Google Scholar]
- 32.Rabey JM., Neufeld MY., Treves TA., Sifris P., Korczyn AD. Cognitive effects of scopolamine in dementia. . J Neural Transm Gen Sect. 1996;103:873–881. doi: 10.1007/BF01273365. [DOI] [PubMed] [Google Scholar]
- 33.Tariot PN., Patel SV., Henderson RE. Age-related decline in central cholinergic function demonstrated with scopolamine. . Psychopharmacology. 1996;125:50–56. doi: 10.1007/BF02247392. [DOI] [PubMed] [Google Scholar]
- 34.Barker A., Jones R., Prior J., Wesnes K. Scopolamine-induced cognitive impairment as a predictor of cognitive decline in healthy elderly volunteers: a 6-year follow-up. . Int J Geriat Psychiatry. 1998;13:244–247. doi: 10.1002/(sici)1099-1166(199804)13:4<244::aid-gps764>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Ghoneim MM., Mewaldt SP. Studies on human memory: the interactions of diazepam, scopolamine and physostigmine. . Psychopharmacology. 1977;52:1–6. doi: 10.1007/BF00426592. [DOI] [PubMed] [Google Scholar]
- 36.Anisman H. Time-dependent changes in activity, reactivity, and responsivity during shock: effects of cholinergic and catecholaminergic manipulations. . Neurology. 1977;27:783–790. doi: 10.1016/s0091-6773(77)92215-5. [DOI] [PubMed] [Google Scholar]
- 37.Liljequist R., Mattila MJ. Effect of physostigmine and scopolamine on the memory functions of chess players. . Med Biol. 1979;57:42–405. [PubMed] [Google Scholar]
- 38.Mewaldt SP., Ghoneim MM. The effects and interactions of scopolamine and methamphetamine on human memory. . Pharmacol Biochem Behav. 1979;10:205–210. doi: 10.1016/0091-3057(79)90088-1. [DOI] [PubMed] [Google Scholar]
- 39.Preston GC., Ward C., Lines CR., Poppleton P., Haigh JR., Traub M. Scopolamine and benzodiazepine models of dementia: cross reversals by RO 151788 and physostigmine. . Psychopharmacology. 1989;98:487–494. doi: 10.1007/BF00441947. [DOI] [PubMed] [Google Scholar]
- 40.Wesnes KA., Simpson PM., White L., et al. Cholinesterase inhibition in the scopolamine model of dementia. . Ann N Y Acad Sci. 1991; 640:268–271. doi: 10.1111/j.1749-6632.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 41.Mohs RC., Davis KL. Interaction of choline and scopolamine in human memory. . Life Sci. 1985;37:193–197. doi: 10.1016/0024-3205(85)90423-0. [DOI] [PubMed] [Google Scholar]
- 42.Wesnes KA., Simpson PM., Christmas L., Anand R., McClelland GR. The effects of moclobemide on cognition. . J Neural Transm. 1989;28(suppl):91–102. [PubMed] [Google Scholar]
- 43.Wesnes K., Anand R., Lorscheid T. Potential of moclobemide to improve cerebral insufficiency identified using a scopolamine model of aging and dementia. . Acta Psychiatr Scand. 1990;360(suppl):71–72. doi: 10.1111/j.1600-0447.1990.tb05338.x. [DOI] [PubMed] [Google Scholar]
- 44.Patat A., Klein MJ., Surjus A., Hucher M., Granier J. RU 41656 does not reverse the scopolamine-induced cognitive deficit in healthy volunteers. . Eur J Clin Pharmacol. 1991;41:225–231. doi: 10.1007/BF00315434. [DOI] [PubMed] [Google Scholar]
- 45.Canal N., Franceschi M., Alberoni M., Castiglioni C., De Moliner P., Longoni A. Effect of L-alpha-glycerylphosphorylcholine on amnesia caused by scopolamine. . IntJ Clin Pharmacol Ther Toxicol. 1991;29:103–107. [PubMed] [Google Scholar]
- 46.Preda L., Alberoni M., Bressi S., et al. Effects of acute doses of oxiracetam in the scopolamine model of human amnesia. . Psychopharmacology. 1993;110:421–426. doi: 10.1007/BF02244648. [DOI] [PubMed] [Google Scholar]
- 47.Wesnes K., Anand R., Simpson P., Christmas L. The use of a scopolamine model to study the potential nootropic effects of aniracetam and piracetam in healthy volunteers. . Int J Geriatr Psychiatry. 1990;6:95–102. doi: 10.1177/026988119000400406. [DOI] [PubMed] [Google Scholar]
- 48.Wesnes K., Simpson PM., Kidd AG. The use of a scopolamine model to study the nootropic effects of tenilsetam (CAS 997) in man. . Med Sci Res. 1987;15:1063–1064. [Google Scholar]
- 49.Shrotriya R., Stallone F., Robinson D., Brady M., Temple D. Clinical pharmacology of BMY 21 502 - a potential cognitive enhancer. Abstracts of the 17th CINP, Kyoto, Japan. 1990 Sep 10-14;Vol II: 230. [Google Scholar]
- 50.Jones RW., Wesnes KA., Kirby J. Effects of NMDA modulation in scopolamine dementia. . Ann N Y Acad Sci. 1991;640:241–244. doi: 10.1111/j.1749-6632.1991.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 51.Brass EP., Polinsky R., Sramek JJ., et al. Effects of the cholinomimetic SDZ ENS-163 on scopolamine-induced cognitive impairment in humans. . J Clin Psychopharmacol. 1995;15:58–62. doi: 10.1097/00004714-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Duka T., Ott H., Rohloff A., Voet B. The effects of a benzodiazepine receptor antagonist beta-carboline ZK-93426 on scopolamine-induced impairment on attention, memory and psychomotor skills. . Psychopharmacology. 1996;123:361–373. doi: 10.1007/BF02246647. [DOI] [PubMed] [Google Scholar]
- 53.Grober E., Gitlin HL., Bang S., Buschke H. Implicit and explicit memory in young, old, and demented adults. . J Clin Exp Neuropsychol. 1992;14:298–316. doi: 10.1080/01688639208402830. [DOI] [PubMed] [Google Scholar]
- 54.Danion JM., Zimmermann MA., Willard-Schroeder D., et al. Effects of scopolamine, trimipramine and diazepam on explicit memory and repetition priming in healthy volunteers. . Psychopharmacology. 1990;102:422–424. doi: 10.1007/BF02244116. [DOI] [PubMed] [Google Scholar]
- 55.Bishop Kl., Curran HV. An investigation of the effects of benzodiazepine receptor ligands and of scopolamine on conceptual priming. . Psychopharmacology. 1998;140:345–353. doi: 10.1007/s002130050775. [DOI] [PubMed] [Google Scholar]
- 56.Rusted JM., Eaton-Williams P., Warburton DM. A comparison of the effects of scopolamine and diazepam on working memory. . Psychopharmacology. 1991;105:442–445. doi: 10.1007/BF02244443. [DOI] [PubMed] [Google Scholar]
- 57.Lydon RG., Nakajima S. Differential effects of scopolamine on working and reference memory depend upon level of training. . Pharmacol Biochem Behav. 1992;43:645–660. doi: 10.1016/0091-3057(92)90206-u. [DOI] [PubMed] [Google Scholar]
- 58.Li HB., Matsumoto K., Tohda M., Yamamoto M., Watanabe H. NMDA antagonists potentiate scopolamine-induced amnestic effect. . Behav Brain Res. 1997;83:225–228. doi: 10.1016/s0166-4328(97)86075-5. [DOI] [PubMed] [Google Scholar]
- 59.Dunne MP. Scopolamine and sustained retrieval from semantic memory. . J Psychopharmacol. 1990;4:13–18. doi: 10.1177/026988119000400103. [DOI] [PubMed] [Google Scholar]
- 60.Eustache F., Agniel A. Neuropsychologie clinique des démences: évaluation et prise en charge. Marseille, France: Solal. 1995 [Google Scholar]
- 61.Fleischman DA., Gabrieli JDE., Reminger SL., Vaidya CJ., Bennett DA. Object decision priming in Alzheimer's disease. . J int Neuropsychol Soc. 1998;4:435–446. doi: 10.1017/s1355617798455036. [DOI] [PubMed] [Google Scholar]
- 62.Christensen H., Griffiths K., Mackinnon A., Jacomb P. A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. . J Int Neuropsychol Soc. 1997;3:631–651. [PubMed] [Google Scholar]
- 63.Ergis AM., Van der Linden M., Deweer B. Explicit memory, procedural learning and lexical priming in Alzheimer's disease. . Cortex. 1994;30:113–126. doi: 10.1016/s0010-9452(13)80327-9. [DOI] [PubMed] [Google Scholar]
- 64.Ebert U., Kirch W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. . Eur J Clin invest. 1998;28:944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 65.Grasby PM., Frith CD., Paulesu E., Friston KJ., Frackowiak RS., Dolan RJ. The effect of the muscarinic antagonist scopolamine on regional cerebral blood flow during the performance of a memory task. . Exp Brain Res. 1995;104:337–348. doi: 10.1007/BF00242019. [DOI] [PubMed] [Google Scholar]
- 66.Cohen RM., Gross M., Semple WE., Nordahl TE., Sunderland T. The metabolic brain pattern of young subjects given scopolamine. . Exp Brain Res. 1994;100:133–143. doi: 10.1007/BF00227285. [DOI] [PubMed] [Google Scholar]
- 67.Blin J., Piercey MF., Giuffra ME., Mouradian MM. Metabolic effects of scopolamine and physostigmine in human brain measured by positron emission tomography . J Neurol Sci. 1994; 123:44–51. doi: 10.1016/0022-510x(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 68.Molchan SE., Matochik JA., Zametkin AJ., et al. A double FDG/PET study of the effects of scopolamine in older adults. . Neuropsychopharmacology. 1994;10:191–198. doi: 10.1038/npp.1994.21. [DOI] [PubMed] [Google Scholar]
- 69.Sunderland T., Esposito G., Molchan SE., Coppola R. Differential cholinergic regulation in Alzheimer's patients compared to controls following chronic blockade with scopolamine: a SPECT study. . Psychopharmacology. 1995;121:231–241. doi: 10.1007/BF02245634. [DOI] [PubMed] [Google Scholar]
- 70.Anis NA., Berry SC., Burton NR., Lodge D. The dissociative anesthetics, ketamine and phencyclidine, selectively decrease excitation of central neurons by N-methyl-D-aspartate. . Br J Pharmacol. 1983;83:179–185. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamakura T., Mori H., Masaki H., Shimoji K., Moshina M. Differential sensitivities of NMDA receptor channel subtypes to non-competitive antagonists. . Neuroreport. 1993;4:687–690. doi: 10.1097/00001756-199306000-00021. [DOI] [PubMed] [Google Scholar]
- 72.Kornbuber J., Wiltfang J. The role of glutamate in dementia. . J Neural Transm. 1998;53(suppl):277–287. doi: 10.1007/978-3-7091-6467-9_24. [DOI] [PubMed] [Google Scholar]
- 73.Perl TM., Bedard L., Kosatsky T., Hockin JC., Todd ECD., Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. . N Engl J Med. 1990;322:1775–1780. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- 74.Masliah E., Alford M., DeTeresa R., Mallory M., Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. . Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 75.Li S., Mallory M., Alford M., Tanaka S., Masliah E. Glutamate transporteralterations in Alzheimer's disease are possibly associated with abnormal APP expression. . J Neuropathol Exp Neurol. 1997;56:901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 76.Tekin S., Aykut-Bingôl C., Tanridag T., Aktan S. Antiglutamatergic therapy in Alzheimer's disease-effects of lamotrigine. . J Neural Transm. 1998;105:295–303. doi: 10.1007/s007020050059. [DOI] [PubMed] [Google Scholar]
- 77.Panegyres PK. The effects of excitotoxicity on the expression of the amyloid precursor protein gene in the brain and its modulation by neuroprotective agents. . J Neural Transm. 1998;105:463–478. doi: 10.1007/s007020050070. [DOI] [PubMed] [Google Scholar]
- 78.Gôrtelmeyer R., Erbler H. Memantine in the treatment of mild-to-moderate dementia syndrome. A double-blind placebo-controlled study. . Drug Res. 1992;42:904–913. [PubMed] [Google Scholar]
- 79.Tanaka S., Liu L., Kimura J., et al. Age-related changes in the proportion of amyloid precursor protein mRNAs in Alzheimer's disease and other neurological disorders. . Brain Res Mol Brain Res. 1992;15:303–310. doi: 10.1016/0169-328x(92)90122-r. [DOI] [PubMed] [Google Scholar]
- 80.Johnson SA., McNeill T., Cordell B., Finch CE. Relation of neuronal APP751/APP-695 mRNA ratio and neuritic plaque density in Alzheimer's disease. . Science. 1990;2:854–857. doi: 10.1126/science.2111579. [DOI] [PubMed] [Google Scholar]
- 81.Mattson MP., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel RE. β-Amy-loid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. . J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klegeris A., McGeer PL. Beta-amyloid protein enhances macrophage production of oxygen free radicals and glutamate. . J Neurosci Res. 1997;49:229–235. doi: 10.1002/(sici)1097-4547(19970715)49:2<229::aid-jnr11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 83.Esclaire F., Lesort M., Blanchard C., Hugon J. Glutamate toxicity enhances tau gene expression in neuronal cultures. . J Neurosci Res. 1997;49:309–318. doi: 10.1002/(sici)1097-4547(19970801)49:3<309::aid-jnr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 84.De Boni U., McLachlan DRC. Controlled induction of paired helical filaments of the Alzheimer type in cultured human neurons, by glutamate and aspartate. . J Neurol Sci. 1985;68:105–118. doi: 10.1016/0022-510x(85)90093-0. [DOI] [PubMed] [Google Scholar]
- 85.Harris JA., Biersner RJ., Edwards D., Bailey LW. Attention, learning, and personality during ketamine emergence: a pilot study. . Anesth Analg. 1975;54:169–172. [PubMed] [Google Scholar]
- 86.Pandit SK., Kothary SP., Kumar S. Low dose intravenous infusion technique with ketamine. . Anesthesia. 1980;35:669–675. doi: 10.1111/j.1365-2044.1980.tb03882.x. [DOI] [PubMed] [Google Scholar]
- 87.Ghoneim MM., Hinrichs JV., Mewaldt SP., Petersen RC. Ketamine: behavioral effects of subanesthetic doses. . J Clin Psychopharmacol. 1985;5:70–77. [PubMed] [Google Scholar]
- 88.Oye I., Paulsen O., Maurset A. Effects of ketamine on sensory perception: evidence for a role of W-methyl-D-aspartate receptors. . J Pharmacol Exp Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- 89.Krystal JH., Karper LP., Seibyl JP., et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. . Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 90.Krystal JH., Karper LP., Bennet A., et al. Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. . Psychopharmacology. 1998;135:213–229. doi: 10.1007/s002130050503. [DOI] [PubMed] [Google Scholar]
- 91.Krystal JH., D'Souza DC., Karper LP., et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. . Psychopharmacology. 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- 92.Malhotra AK., Finals DA., Weingartner H., et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. . Neuropsychopharmacology. 1996; 14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 93.Adler CM., Goldberg TE., Malhotra AK., Pickar D., Breier A. Effects of ketamine on thought disorder, working memory and semantic memory in healthy volunteers. . Biol Psychiatry. 1998;43:811–816. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- 94.Newcomer JW., Farber NB., Jevtovic-Todorovic V., et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. . Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 95.Sunderland T., Molchan SE., Little JT., Bahro M., Putnam KT., Weingartner H. Pharmacologic challenges in Alzheimer disease and normal controls: cognitive modeling in humans. . Alzheimer Dis Assoc Disord. 1997;11(suppl 4):S23–S26. [PubMed] [Google Scholar]
- 96.Little JT., Johnson DN., Minichiello M., Weingartner H., Sunderland T. Combined nicotinic and muscarinic blockade in elderly normal volunteers: cognitive, behavioral and physiologic responses. . Neuropsychopharmacology. 1998;19:60–69. doi: 10.1016/S0893-133X(98)00002-5. [DOI] [PubMed] [Google Scholar]
- 97.Broocks A., Little JT., Martin A., et al. The influence of ondansetron and mchlorophenylpiperazine on scopolamine-induced cognitive, behavioral, and physiological responses in young healthy controls. . Biol Psychiatry. 1998;43:408–416. doi: 10.1016/s0006-3223(97)00388-0. [DOI] [PubMed] [Google Scholar]
- 98.Vitiello B., Martin A., Hill J., et al. Cognitive and behavioral effects of cholinergic, dopaminergic, and serotonergic blockade in humans. . Neuropsychopharmacology. 1997;16:15–24. doi: 10.1016/S0893-133X(96)00134-0. [DOI] [PubMed] [Google Scholar]
- 99.Gomez-lsla T., Hyman BT. Connection and cognitive impairment in Alzheimer's disease. In: Hyman BT, Duyckaerts C, Christen Y, eds. . Connections, Cognition and Alzheimer's Disease (Research and Perspectives in Alzheimer's Disease). Berlin, Germany: Springer-Verlag. 1997:149–166. [Google Scholar]
- 100.Ladner CJ., Lee JM. Pharmacological drug treatment of Alzheimer disease: the cholinergic hypothesis revisited. . J Neuropathol Exp Neurol. 1998;57:719–731. doi: 10.1097/00005072-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Auld DS., Kar S., Quirion R. P-Amyloid peptides as direct cholinergic neuromodulators: a missing link? . Trends Neurosci. 1998;21:43–49. doi: 10.1016/s0166-2236(97)01144-2. [DOI] [PubMed] [Google Scholar]
- 102.Yoshikawa K. Neurotoxicity of amyloid p-protein and the amyloid pprotein precursor. In: Goate A, Ashall F, eds. . Pathobiology of Alzheimer's Disease. London, UK: Academic Press. 1995:145–165. [Google Scholar]
- 103.Kar S., Seto D., Gaudreau P., Quirion R. p-Amyloid-related peptides inhibit potassium-evoked acetylcholine release from rat hippocampal formation. . J Neurosci. 1996;16:1034–1040. doi: 10.1523/JNEUROSCI.16-03-01034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kar S., Issa AM., Seto D., Auld DS., Collier B., Quirion R. Amyloid p-peptide inhibits high-affinity choline uptake and acetylcholine release in rat hippocampal slices. . J, Neurochem. 1998;70:2179–2187. doi: 10.1046/j.1471-4159.1998.70052179.x. [DOI] [PubMed] [Google Scholar]
- 105.Pedersen WA., Kloczewiak MA., Blusztajn JK. Amyloid beta protein reduces acetylcholine synthesis in a cell line derived from cholinergic neurons of the basal forebrain. . Proc Natl Acad Sci USA. 1996;93:8068–8071. doi: 10.1073/pnas.93.15.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoshi M., Takashima A., Muramaya M., et al. Non-toxic amyloid beta peptide 1-42 suppresses acetylcholine synthesis. Possible role in cholinergic dysfunction in Alzheimer's disease. . J Biol Chem. 1997;272:2038–2041. doi: 10.1074/jbc.272.4.2038. [DOI] [PubMed] [Google Scholar]
- 107.Sheu KFR., Kim YT., Blass JP., Weksler ME. An immunohistochemical study of the pyruvate dehydrogenase deficit in Alzheimer's disease brain. . Ann Neurol. 1985;17:444–449. doi: 10.1002/ana.410170505. [DOI] [PubMed] [Google Scholar]
- 108.Maire JCE., Wurtman RJ. Choline production from choline-containing phospholipids: a hypothetical role in Alzheimer's disease and aging. . Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:637–642. doi: 10.1016/0278-5846(84)90027-7. [DOI] [PubMed] [Google Scholar]
- 109.Pavia J., de Ceballos ML., de la Cuesta FS. Alzheimer's disease: relationship between muscarinic cholinergic receptors, p-amyloid and tau proteins. . Fundam Clin Pharmacol. 1998;12:473–481. doi: 10.1111/j.1472-8206.1998.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 110.Whitehouse PJ., Martino AM., Marcus KA., et al. Reductions in acetylcholine and nicotinic binding in several degenerative diseases. . Ann Neurol. 1988;45:722–724. doi: 10.1001/archneur.1988.00520310028012. [DOI] [PubMed] [Google Scholar]
- 111.Nordberg A., Alafuzoff I., Windblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. . J Neurosci Res. 1992;31:103–111. doi: 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- 112.Buxbaum JD., Oishi M., Chen HI., et al. Cholinergic agonists and interleukin-1 regulate processing and secretion of Alzheimer β4 amyloid protein precursor. . Proc Natl Acad Sci U S A. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nitsch RM., Slack BE., Wurtman RJ., Gordon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. . Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 114.Greenwood AF., Powers RE., Jope RS. Phosphoinositide hydrolysis, G alpha q, phospholipase C, and protein kinase C in post mortem human brain: effects of post mortem interval, subject age, and Alzheimer's disease. . Neuroscience. 1995;69:125–138. doi: 10.1016/0306-4522(95)00220-d. [DOI] [PubMed] [Google Scholar]
- 115.Jope RS., Song L., Power RE. Cholinergic activation of phosphoinositide signalling is impaired in Alzheimer's disease brain. . Neurobiol Aging. 1997;18:111–120. doi: 10.1016/s0197-4580(96)00205-9. [DOI] [PubMed] [Google Scholar]
- 116.Flynn DD., Weinstein DA., Mash DC. Loss of high-affinity agonist binding to M, muscarinic receptors in Alzheimer's disease: implication for the failure of cholinergic replacement therapies. . Ann Neurol. 1991;29:256–262. doi: 10.1002/ana.410290305. [DOI] [PubMed] [Google Scholar]
- 117.Kelly JF., Furukawa K., Barger SW., et al. Amyloid beta-peptide disrupts carbachol-induced muscarinic cholinergic signal transduction in cortical neurons. . Proc Natl Acad Sci U SA. 1996;93:6753–6758. doi: 10.1073/pnas.93.13.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qian N., Russell M., Johnson GL. Acetylcholine muscarinic receptor regulation of the Ras/Raf/MAP kinase pathway. . Life Sci. 1995;56:945–949. doi: 10.1016/0024-3205(95)00032-2. [DOI] [PubMed] [Google Scholar]
- 119.Sadot E., Gurwitz D., Barg J., Behar L., Ginzburg I., Fisher A. Activation of m, muscarinic acetylcholine receptor regulates the tau phosphorylation in transfected PC12 cells. . J Neurochem. 1996;66:877–880. doi: 10.1046/j.1471-4159.1996.66020877.x. [DOI] [PubMed] [Google Scholar]
- 120.Schliebs R., Ressner S. Distribution of muscarinic acetylcholine receptors in the CNS. In: Stone TW, ed. CMS . Neurotransmitters and Neuromodulators: Acetylcholine. London, UK: CRC Press. 1995:67–83. [Google Scholar]
- 121.Moriya H., Takagi Y., Nakanishi T., Hayashi M., Tani T., Hirotsu I. Affinity profiles of various muscarinic antagonists for cloned human muscarinic acetylcholine receptor (MACHR) subtypes and MACHRS in rat heart and submandibular gland. . Life Sci. 1999;64:2351–2358. doi: 10.1016/s0024-3205(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 122.Jaup BH., Blomstrand C. Cerebrospinal fluid concentrations of pirenzepine after therapeutic dosage. . ScandJ Gastroenterol. 1980;15(suppl 66):35–37. [PubMed] [Google Scholar]
- 123.Stacher G., Bauer P., Schmierer G., Steinringer H. The effect of intramuscular pirenzepine on oesophageal contractile activity and lower oesophageal sphincter pressure under fasting conditions and after a standard meal. A double-blind study. . IntJ Clin Pharmacol Biopharm. 1979;17:442–448. [PubMed] [Google Scholar]
- 124.Fink M., Irwin P. EEG and behavioral effects of pirenzepine in normal volunteers. . Scand J Gastroenterol. 1980;66(suppl):39–46. [PubMed] [Google Scholar]
- 125.Walters L., Bartel P., Sommers DK., Becker P. The effects of anticholinergics on the photopalpebral reflex, memory and mood. . Methods Find Exp Clin Pharmacol. 1988;10:419–425. [PubMed] [Google Scholar]
- 126.Moore CL., Wolfe M. Inhibition of p-amyloid formation as a therapeutic strategy. . Exp Opin Ther Patents. 1999;9:135–146. [Google Scholar]
- 127.Soto C. Plaque busters: strategies to inhibit amyloid formation in Alzheimer's disease. . Mol Med Today. 1999;5:343–350. doi: 10.1016/s1357-4310(99)01508-7. [DOI] [PubMed] [Google Scholar]
- 128.Schenk D., Barbour R., Dunn W., et al. Immunization with amyloid-p attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 129.Molchan SE., Mellow AM., Lawlor BA., et al. TRH attenuates scopolamine-induced memory impairment in humans. . Psychopharmacology. 1990;100:84–89. doi: 10.1007/BF02245795. [DOI] [PubMed] [Google Scholar]
- 130.Gibbs RB., Burke AM., Johnson DA. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. . Horm Behav. 1998;34:112–125. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- 131.Silva RH., Felicio LF., Frussa-Filho R. Ganglioside GM1 attenuates scopolamine-induced amnesia in rats and mice. . Psychopharmacology. 1999;141:111–117. doi: 10.1007/s002130050814. [DOI] [PubMed] [Google Scholar]
- 132.Eglen RM., Choppin A., Dillon MP., Hegde S. Muscarinic receptor ligands and their therapeutic potential. . Curr Opin Chem Biol. 1999;3:426–432. doi: 10.1016/S1367-5931(99)80063-5. [DOI] [PubMed] [Google Scholar]