Abstract
Since the time of Kraepelin and Bleuler, it has been recognized that schizophrenia is associated with a profound and persistent cognitive impairment. This paper reviews the major clinical and epidemiological studies of cognitive functioning in schizophrenia and other psychotic disorders, and presents several possible models to explain the association between cognitive impairment and psychosis. Cognitive impairment is present in the majority of patients with schizophrenia, and, in some, it is already evident in the premorbid stages of the disorder. This cognitive impairment is not secondary to psychotic symptoms, negative symptoms, or socioeconomic status. Cognitive impairment can also be observed in nonpsychotic family members of psychotic patients. On the basis of this evidence, it has been proposed that abnormal cognitive functioning can be considered as a possible causal risk û chosis. Recent studies assessing the relations genetic background, cognition, brain functic ophrenia are presented here as an outline for future research.
Keywords: cognitive function, schizophrenia, risk factor, genetics, epidemiological study
Abstract
Desde los tiempos de Kraepelin y Bieuler se ha reconocido que la esquizofrenia se asocia con un profundo y persistente deterioro cognitivo. Este artículo revisa los principales estudios clínicos y epidemiológicos acerca del funcionamiento cognítivo en la esquizofrenia y otros trastornos psicóticosf y presenta varios modelos posibles para explicar la asociación entre deterioro cognitivo y psicosis. El deterioro cognitivo está presente en la mayoría de los pacientes con esquizofrenia y en algunos resulta evidente en las etapas premórbidas del trastorno. Este deterioro cognitivo no es secundario a ios síntomas psicóticos, a los síntomas negativos ni al nivel socioeconómico. El deterioro cognitivo también puede observarse en familiares no psicóticos de los pacientes psicóticos. En base a esta evidencia se ha propuesto que el funcionamiento cognitivo anormal puede ser considerado como un posible factor de riesgo causal para la psicosis. En esta revisión se presentan estudios recientes que evalúan la relación entre la base genética, la cognición, el funcionamiento cerebral y la esquizofrenia como esbozos para futuras investigaciones.
Abstract
Depuis l'époque de Kraepelin et Bleuler, il est reconnu que la schizophrénie est associée avec un déficit cognitif profond et durable. Cet article passe en revue les principales études cliniques et épidémiologiques du fonctionnement cognitif dans la schizophrénie et dans d'autres troubles psychotiques, et présente plusieurs modèles possibles pour expliquer l'association entre déficit cognitif et psychose. Un déficit cognitif est présent chez la plupart des patients atteints de schizophrénie et, chez certains, il est déjà évident au cours des stades pré-morbides de la pathologie. Ce déficit cognitif n'est pas la conséquence des symptômes psychotiques, des symptômes négatifs ou du statut socio-économique. Le déficit cognitif peut aussi s'observer chez des membres non psychotiques de la famille de patients psychotiques. Sur la base de cet argument il a été suggéré qu'un fonctionnement cognitif anormal pourrait être un possible facteur de risque causal de psychose. Des études récentes évaluant la relation entre terrain génétique, cognition, fonction cérébrale et schizophrénie sont présentées ici traçant ainsi les grandes lignes de la recherche future.
A great deal of speculation and research has been devoted to determining whether a relationship exists between intellectual performance and schizophrenia. Kraepelin1 and Bleuler2 recognized at the beginning of the 20th century that deterioration in intellectual performance is a characteristic of schizophrenia patients. More recently, the study of cognitive deficits in schizophrenia has been expanding rapidly; over just one decade in the 1990s, the annual publication rate for studies of cognition and schizophrenia increased almost fivefold.3 Considerable evidence indicates that cognitive deficits are a core impairment of schizophrenia, including crosssectional and longitudinal studies of cognitive functioning of first-episode and chronic schizophrenia patients, studies of the nonpsychotic relatives of schizophrenia patients, studies of premorbid cognitive performance in individuals destined to develop schizophrenia, and genetic studies that incorporate assessment of intermediate phenotypes in patients. The focus of this paper will be on the magnitude and frequency of the cognitive impairment in schizophrenia, its origins and relationship to symptoms, and the relationship between the genetic basis of schizophrenia and cognition.
Cogniive functioning in schizophrenia patients
A large body of evidence demonstrates that cognitive deviation from population norms is very common among patients with chronic schizophrenia. Between 75% and 85% of all schizophrenia patients exhibit severe abnormal cognitive functioning.4,5 Schizophrenia patients have a marked deficit in general intellectual ability, or IQ, scoring an average of 19 IQ points below controls.*(For reference throughout the text: a difference in IQ by a magnitude of one standard deviation corresponds to 15 IQ points.)6 The deficit in general intellectual ability is coupled with abnormalities in specific neuropsychological functions, particularly abnormal memory working memory attention, and executive functioning.5-8
Studies demonstrate that the cognitive deficits (in both IQ and specific neuropsychological functions) are already evident in patients after the first psychotic episode, and are not due to neuroleptic medication. Saykin et al8 studied 37 patients who were never exposed to neuroleptic medication. Patients showed a broad range of neuropsychological deficits, including deficits in attention, abstraction, memory, and learning. Patients performed one to three standard deviations below controls. Mohamed et al9 studied 94 first-episode patients, of whom the majority were neuroleptic-naive. The patients performed between one to two standard deviations below controls on the majority of cognitive measures, which included measures of IQ, memory, attention, and executive functions. Results are similar after treatment with neuroleptics, although the cognitive deficit may be slightly milder. Bilder et al10 administered a comprehensive neuropsychological test battery to 94 first-episode patients after initial stabilization of psychosis. Patients were impaired on all 41 measures, with the majority of deficits exceeding one standard deviation.
Prospective longitudinal studies of schizophrenia patients following the first episode have suggested stabilization and even mild improvement over time. Censits et al11 followed 30 first-episode patients for 19 months. Patients remained one to two standard deviations below controls on all neuropsychological measures, with no significant change in performance over time. In another follow-up study 35 first-episode or recent-onset schizophrenia patients were administered a comprehensive battery of neuropsychological tests during index hospitalization and at either 1- or 2-year follow-up examination.12 Neuropsychological deficits remained stable in most domains. Studies with longer duration of follow-up show similar results. Gold et al13 studied 54 first-episode or recent-onset schizophrenia patients and followed them for 5 years. They found a modest, 3-point improvement in IQ, especially in performance IQ. More specifically 37 subjects improved their performance, 13 subjects performed worse, and 4 subjects showed no change. Change was also evident on specific neuropsychological measures: attention, memory, and executive functions slightly improved, and motor speed deteriorated. DeLisi et al14 evaluated the longitudinal neuropsychological performance of 20 first-episode patients at index hospitalization and at 4-year follow-up. The majority of the neuropsychological test scores did not change over time; improvement occurred in tests of concentration and speed, and overall global functioning. Hoff et al15 studied language, executive, memory processing speed, and sensory-perceptual functions in 42 patients with a first hospitalization for schizophrenia or schizophreniform disorder at approximate yearly intervals for the first 2 to 5 years of illness. Patients consistently scored one to two standard deviations below normal comparison subjects on neuropsychological test measures during the 5-year course of the study Patients exhibited some improvement on measures of language, executive functions, and a mild decline in verbal memory.
In their meta-analysis of neuropsychological performance in schizophrenia, Heinrichs and Zakzanis6 found no significant relationships between IQ deficits and potential moderators, including symptom severity, age, education, neuroleptic dose, sample gender composition, or age of illness onset. The correlations between specific neuropsychological functions and measures of psychopathology, such as positive or negative symptoms, are also relatively modest.8 In line with this observation, Mohamed et al9 and Bilder et al10 reported that worse neuropsychological performance was only weakly correlated with severity of negative symptoms and neuroleptic dose.
Cognitive functioning in relatives of schizophrenia patients
Numerous studies have demonstrated that relatives of patients with schizophrenia exhibit cognitive impairments that are milder than, yet similar to, those seen in schizophrenia patients. Studies of monozygotic twins discordant for schizophrenia have found that the nonpsychotic discordant twins manifested subtle impairments in memory attention, abstraction, and IQ.16-18 Studies of siblings of patients with schizophrenia yielded similar results: both schizophrenia patients and their nonschizophrenic siblings were cognitively impaired compared with normal controls, and the nonschizophrenic siblings had a performance that was intermediate between the probands and normal controls. The cognitive deficits of the relatives included impairments in IQ and specific abnormalities in memory, attention, and abstraction.19-23 These cognitive deficits in relatives were stable over time.24 Given that the cognitive abnormalities are also evident in the nonschizophrenic siblings, these abnormalities do not appear to be a result of longstanding illness; rather, they appear to represent longlasting markers of risk for the disorder, and possibly indicate a genetic predisposition.
The origins of the cognitive impairment in schizophrenia
It is clear from the studies reviewed above that schizophrenia is coupled with a severe and debilitating cognitive impairment, which is persistent and independent of positive or negative symptoms, and does not respond to neuroleptic treatment. However, cognitive impairments are also found in other, nonpsychotic psychiatric disorders, though they may be less severe than those seen in schizophrenia.25-27 Thus, a number of investigators have sought to determine whether lower cognitive functioning occurs as a precursor to, or risk factor for, schizophrenia or only occurs concomitantly with the overt expression of the illness. A review of these early studies, mainly in clinical samples, can be found in Alyward et al.28 Below, I shall describe the main epidemiological studies addressing the relationship between childhood and adolescence cognitive functioning and later schizophrenia. Epidemiological samples are representative of the population, and therefore protect against the ascertainment bias that often affects clinical samples.
Schizophrenia In the British 1946 birth cohort
A study of 5362 British subjects born in March 1946 has yielded 30 cases of narrowly defined (Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Reivsed [DSM-III-R] criteria) schizophrenia up to age 43 years.29 Compared with the rest of the cohort, those subjects who went on to develop schizophrenia had lower cognitive functioning. Specifically educational test scores at ages 8, 11, and 15, adjusted for sex and social class, were consistently lower for the preschizophrenia group. Approximately half of future patients were in the lowest tertile of performance on the cognitive tests. Deficits were particularly noted in verbal, nonverbal, and mathematical skills, and were independent of ratings of behavior.
Dunedin multidisciplinary health and development study
This study followed a 1-year birth cohort of 1037 children born in Dunedin, New Zealand, from birth to age 26. 30 The authors found that the 36 future patients (defined as individuals with a schizophreniform disorder) performed worse than controls on standard IQ tests at each of five assessments between age 3 and 11 years. The cognitive deficits were present only among children later diagnosed as having schizophreniform disorder, but not in those later diagnosed with other nonpsychotic psychiatric disorders, and were independent of the effects of socioeconomic and obstetric factors.
Philadelphia cohort of the national collaborative perinatal project
Seventy-two patients with schizophrenia or schizoaffective disorder and 7941 controls with no diagnosis were found in a birth cohort (originally collected between 1959 to 1966) whose members had been evaluated with standardized tests of intelligence at 4 and 7 years of age.31 Adult psychiatric morbidity was ascertained via a longitudinal treatment database indexing regional public health service utilization, and diagnoses were made by review of all pertinent medical records according to DSM-IV criteria. Patients with schizophrenia performed significantly worse than controls on IQ tests at 4 and 7 years of age.
Schizophrenia in the 1949 to 1950 Swedish conscript study
Another cohort comprised of some 50 000 Swedish male conscripts.32 Baseline assessment of cognitive functioning was collected at conscription. Cases were identified over 13 years from the Swedish national register of psychiatric care (International Classification of Diseases, 8th Edition [ICD-8] diagnoses) . One hundred and ninety-five cases of schizophrenia and 192 cases of other psychoses were identified. Cognitive testing at age 18 showed a significant relationship between low IQ scores and later schizophrenia. A similar, yet weaker, association was observed for cases with other psychoses. Cognitive deficits were independent of ratings of social withdrawal, socioeconomic status, drug abuse, or family history of psychiatric disorders.
Schizophrenia in the Israeli conscript study
A case-control study on schizophrenia was nested within a cohort of male conscripts into the Israeli army between 1985 and 1991.33,34 Data from cognitive tests at conscription were linked to a national case register (ICD-10 diagnoses) up to 1995, resulting in a comparison between 509 men healthy at conscription but who later developed schizophrenia, and 9215 men who did not appear in the register, matched according to age at follow-up and the type of school attended. Deficits in cognitive functioning assessed at conscription strongly predicted later schizophrenia. Cognitive deficits were independent of ratings of social withdrawal. Premorbid cognitive deficits were evident in future schizoaffective patients, but not in those with a diagnosis of nonaffective psychosis.
Summary of studies of cognitive impairment and schizophrenia
Despite differences in methods of case ascertainment, use of diverse cognitive tests, and differences in times when the cohorts were established and geographical areas, the studies described above consistently show that low premorbid cognitive functioning is associated with increased risk for schizophrenia and other psychotic disorders. It is, however, unclear whether these manifestations are themselves part of a causal pathway to psychosis, or merely indicate vulnerability
Is cognitive impairment a causal risk factor?
How then should we account for the observed relationship between low cognitive functioning and psychosis? A dominant view about the etiology of schizophrenia is the so-called neurodevelopmental hypothesis, suggesting that schizophrenia arises from early, possibly fetal abnormalities of genetic and/or environmental origin.35,36 David et al32 discussed three possible explanations corresponding to the neurodevelopmental hypothesis: low cognitive functioning may be a confounder, may be an independent cause, or may indeed lie on the causal pathway.
Confounding factor
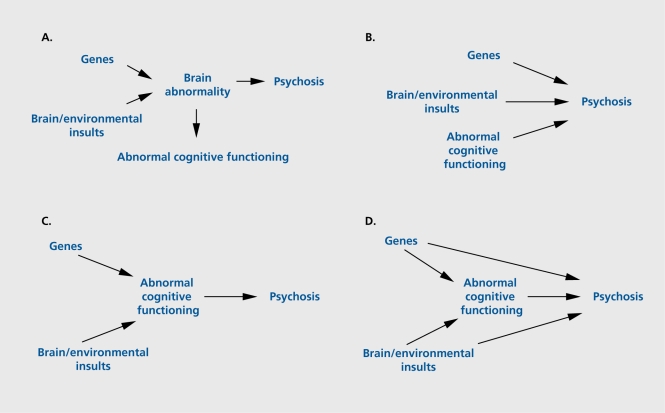
It might be argued that the abnormal functioning merely reflects the prodrome to the illness. However, this is highly unlikely since the association between low cognitive functioning and schizophrenia was evident even in individuals first admitted up to 10 years after assessment. Furthermore, no association has been found between age of onset and the severity of premorbid cognitive impairment.30,32,33 Alternatively, genes predisposing to psychosis and/or early brain damage may cause brain abnormalities, which, among other things, also cause abnormal cognitive functioning (Figure 1A). A number of conditions that impair cognitive functioning are also associated with increased risk of psychotic symptoms, including Huntington's disease, Alzheimer's disease, traumatic brain injury multiple sclerosis, and epilepsy37 Furthermore, cognitive functioning shows a relationship with other possible risk factors for schizophrenia, such as low birth weight.38,39 Twin and family studies,16,23 however, indicate that, even when the effects of genetic risk and prenatal environment are controlled, the association between abnormal cognitive functioning and psychosis remains. Cannon et al30 further demonstrated that the low premorbid cognitive functioning in future patients was independent of the effects of obstetric factors.
Figure 1. Diagrams illustrating relationships between cognitive function and psychosis: (A) abnormal cognitive functioning as a confounder; (B) independent causes, including abnormal cognitive functioning, associated with the development of psychosis; (C) causes of psychosis with abnormal cognitive functioning lying on a causal pathway; and (D) abnormal cognitive functioning lying on a causal pathway, but other direct pathways also operate.
Independent cause
Abnormal cognitive functioning itself might increase the risk for later psychosis. Individuals with mental handicap are at increased risk of psychosis.40 However, considering that, in the general population, most individuals with abnormal cognitive functioning do not develop psychosis (ie, abnormal cognitive functioning has poor positive predictive value), other risk factors must also be involved (Figure 1B).
Causal pathway
Most, if not all, putative risk factors for schizophrenia show a relationship with lower cognitive performance. So-called “high-risk” studies have consistently reported that children of patients with schizophrenia perform worse on intelligence tests than children of nonschizophrenic parents.28,41,42 Obstetric and birth complications are another example.38 Thus, the third model suggests that abnormal cognitive functioning could be the means by which other genetic and/or environmental influences increase the risk for psychosis (Figure 1C). Since not all schizophrenia patients have cognitive impairment, and a number of different genes may contribute to risk for psychosis, it is likely that there are also direct pathways from genes and the environment to psychosis (Figure 1D).
Cognitive model
The processes described in the previous sections do not, however, offer an explanation of how abnormal cognitive functioning affects the development of psychosis. Abnormal cognitive functioning could interfere with information processing at various levels and domains leading eventually to the psychopathology of schizophrenia. A person's abnormal cognition impairs his or her ability to comprehend the complexities of society, which could lead to misunderstandings, feelings of paranoia, and social withdrawal.32 Deficits in social cognition are certainly well recognized in patients with schizophrenia. Abnormal cognitive processes may also interact with a developmental process induced by genes or environment.43 This abnormal cognitive process will induce behavior that will itself tend to alter the environment in which an individual functions, leading to altered experience and further abnormality in the developmental process.
Genes for cognition and schizophrenia?
The relationship between impaired cognition and schizophrenia has led several investigators to suggest targeting cognitive functioning as an intermediate phenotype (or endophenotype) rather than clinical diagnosis. This would reduce heterogeneity in genetic studies,23 since cognitive abnormalities may be more directly related to the biological effects of susceptibility genes (as proposed by the models in Figures 1C and 1D and discussed in the previous section) .
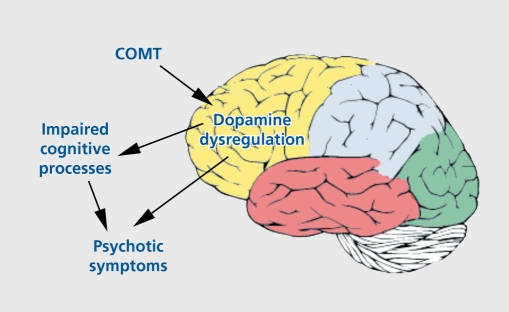
Several research groups are now using endophenotypes to study the genetic basis of schizophrenia and cognition. One especially promising finding is evidence implicating frontal dopamine system dysfunction in the pathogènesis of schizophrenia.44 The gene for catecholamine O-methyltransferase (COMT) codes for one of the major enzymes catalyzing the metabolism of dopamine. It has been mapped to chromosomal region 22q11, and contains a functional polymorphism (Val158Met) that results in two common variants of the enzyme (Val and Met) corresponding to high and low dopamine catabolism, respectively The COMT gene has been examined several times for an association with schizophrenia. Although not conclusive, family-based association studies and case-control studies do support the claim that variability of this gene could constitute a risk factor for schizophrenia, specifically the Val allele.45 Studies of healthy individuals, and schizophrenia patients have further demonstrated that the COMT genotype is related in an allele dosage fashion to performance on tests of working memory and executive functions, with more Met alleles associated with better performance.46-48 Egan et al46 also examined the effect of COMT genotype on prefrontal physiology during a working memory task using functional magnetic resonance imaging (MRI) . Met allele load consistently predicted a more efficient physiological response in prefrontal cortex. Thus, according to these results, the high levels of dopamine in individuals with the Met/Met genotype enhance prefrontal function and therefore cognitive performance, and are also associated with lower risk for psychosis (Figure 2).
Figure 2. Schematic representation of the putative effect of a schizophrenia susceptibility gene (COMT) on neurotransmission and the relationship with cognition and psychosis. COMT polymorphisms effect dopamine regulation in the frontal lobes, through which cognitive processes are effected. Dopamine dysregulation increases risk to psychosis either directly, and/or through impaired cognitive processes. COMT, catecholamine O-methyltransferase.
Conclusion
The evidence reviewed in this paper strongly supports the view that cognitive deficits are a risk factor for schizophrenia and other psychotic disorders. Cognitive deficit is a stable, “trait-like” condition, independent of psychotic symptoms and mostly unaffected by antipsychotic treatments. In some patients, it is evident many years before psychotic symptoms are expressed and, after the onset of psychotic symptoms, cognitive deficits are present in the large majority of patients. Future studies of the genetic basis of specific cognitive functions and the association between genes, cognition, and brain processes will undoubtedly help better understand the role of cognition in the development of psychotic illness.
REFERENCES
- 1.Kraepelin E. Dementia Praecox. Barclay RM, transl. Melbourne, Fla: Robert E. Krieger; 1913 [Google Scholar]
- 2.Bleuler E. Dementia Praecox, or the Group of Schizophrenias (1911). New York, NY: International Universities Press; 1950 [Google Scholar]
- 3.Green MF., Nuechterlein KH., Gold JM., et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Kremen WS., Seidman LJ., Faraone SV., Toomey R., Tsuang MT. The paradox of normal neuropsychological function in schizophrenia. J Abnorm Psychoi. 2000;109:743–752. doi: 10.1037//0021-843x.109.4.743. [DOI] [PubMed] [Google Scholar]
- 5.Palmer BW., Heaton RK., Paulsen JS., et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 6.Heinrichs RW., Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 7.Saykin AJ., Gur RC., Gur RE., et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 8.Saykin AJ., Shtasel DL., Gur RE., et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed S., Paulsen JS., O'Leary D., Arndt S., Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- 10.Bilder RM., Goldman RS., Robinson D., et al. Neuropsychology of firstepisode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 11.Censits DM., Ragland JD., Gur RC., Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophr Res. 1997;24:289–298. doi: 10.1016/s0920-9964(96)00091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nopoulos P., Flashman L., Flaum M., Arndt S., Andreasen N. Stability of cognitive functioning early in the course of schizophrenia. Schizophr Res. 1994;14:29–37. doi: 10.1016/0920-9964(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 13.Gold S., Arndt S., Nopoulos P., O'Leary DS., Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry. 1999;156:1342–1348. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- 14.DeLisi LE., Tew W., Xie S., et al. A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry. 1995;38:349–360. doi: 10.1016/0006-3223(94)00376-e. [DOI] [PubMed] [Google Scholar]
- 15.Hoff AL., Sakuma M., Wieneke M., Horon R., Kushner M., DeLisi LE. Longitudinal neuropsychological follow-up study of patients with firstepisode schizophrenia. Am J Psychiatry. 1999;156:1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg TE., Ragland JD., Torrey EF., Gold JM., Bigelow LB., Weinberger DR. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry. 1990;47:1066–1072. doi: 10.1001/archpsyc.1990.01810230082013. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg TE., Torrey EF., Gold JM., Ragland JD., Bigelow LB., Weinberger DR. Learning and memory in monozygotic twins discordant for schizophrenia. Psychol Med. 1993;23:71–85. doi: 10.1017/s0033291700038861. [DOI] [PubMed] [Google Scholar]
- 18.Cannon TD., Huttunen MO., Lonnqvist J., et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon TD., Zorrilla LE., Shtasel D., et al. Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry. 1994;51:651–661. doi: 10.1001/archpsyc.1994.03950080063009. [DOI] [PubMed] [Google Scholar]
- 20.Keefe RS., Silverman JM., Roitman SE., et al. Performance of nonpsychotic relatives of schizophrenic patients on cognitive tests. Psychiatry Res. 1994;53:1–12. doi: 10.1016/0165-1781(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 21.Egan MF., Goldberg TE., Gscheidle T., Weirich M., Bigelow LB., Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 22.Egan MF., Goldberg TE., Gscheidle T., et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- 23.Kremen WS., Seidman LJ., Pepple JR., Lyons MJ., Tsuang MT., Faraone SV. Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophr Bull. 1994;20:103–119. doi: 10.1093/schbul/20.1.103. [DOI] [PubMed] [Google Scholar]
- 24.Faraone SV., Seidman LJ., Kremen WS., Toomey R., Pepple JR., Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a 4-year follow-up study. J Abnorm Psychol. 1999;108:176–181 . doi: 10.1037//0021-843x.108.1.176. [DOI] [PubMed] [Google Scholar]
- 25.Gourovitch ML., Torrey EF., Gold JM., Randolph C., Weinberger DR., Goldberg TE. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol Psychiatry. 1999;45:639–646. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 26.Murphy FC., Sahakian BJ. Neuropsychology of bipolar disorder. Br J Psychiatry. 2001;178(suppl 41):S120–S127. [PubMed] [Google Scholar]
- 27.Quraishi S., Frangou S. Neuropsychology of bipolar disorder: a review. J Affect Disord. 2002;72:209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 28.Aylward E., Walker E., Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull. 1984;10:430–459. doi: 10.1093/schbul/10.3.430. [DOI] [PubMed] [Google Scholar]
- 29.Jones P., Rodgers B., Murray R., Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 30.Cannon M., Caspi A., Moffitt TE., et al. Evidence for early-childhood, pandevelopmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 31.Cannon TD., Bearden CE., HoIIister JM., Rosso IM., Sanchez LE., Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- 32.David AS., Malmberg A., Brandt L., Allebeck P., Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med. 1997;27:1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- 33.Davidson M., Reichenberg A., Rabinowitz J., Weiser M., Kaplan Z., Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- 34.Reichenberg A., Weiser M., Rabinowitz J., et al. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. Am J Psychiatry. 2002;159:2027–2035. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- 35.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 36.Murray RM., Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummings JL., Trimble MR. Neuropsychiatry and Behavioral Neurology Washington, DC: American Psychiatric Press; 1995 [Google Scholar]
- 38.Cannon M., Jones PB., Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 39.Matte TD., Bresnahan M., Begg MD., Susser E. Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: cohort study. BMJ. 2001;323:310–314. doi: 10.1136/bmj.323.7308.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reid AH. Schizophrenia in mental retardation: clinical features. Res Dev Disabil. 1989;10:241–249. doi: 10.1016/0891-4222(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 41.Erlenmeyer-Kimling L., Cornblatt B. The New York High-Risk Project: a follow-up report. Schizophr Bull. 1987;13:451–461. doi: 10.1093/schbul/13.3.451. [DOI] [PubMed] [Google Scholar]
- 42.Erlenmeyer-Kimling L., Golden RR., Cornblatt BA. A taxometric analysis of cognitive and neuromotor variables in children at risk for schizophrenia. J Abnorm Psychol. 1989;98:203–208. doi: 10.1037//0021-843x.98.3.203. [DOI] [PubMed] [Google Scholar]
- 43.Jones PB., Tarrant CJ. Developmental precursors and biological markers for schizophrenia and affective disorders: specificity and public health implications. Eur Arch Psychiatry Clin Neurosci. 2000;250:286–291 . doi: 10.1007/s004060070003. [DOI] [PubMed] [Google Scholar]
- 44.Davis KL., Kahn RS., Ko G., Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 45.Glatt SJ., Faraone SV., Tsuang MT. Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry. 2003;160:469–476. doi: 10.1176/appi.ajp.160.3.469. [DOI] [PubMed] [Google Scholar]
- 46.Egan MF., Goldberg TE., Kolachana BS., et al. Effect of COMT Val 108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosa A., Peralta V., Cuesta MJ., et al. New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. Am J Psychiatry. 2004;161:1110–1112. doi: 10.1176/appi.ajp.161.6.1110. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra AK., Kestler LJ., Mazzanti C., Bates JA., Goldberg TE., Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]