Abstract
Epidemiological studies show that there is a correlation between chronic depression and the likelihood of demential in later life. There is evidence that inflammatory changes in the brain are pathological features of both depression and dementia. This suggests that an increase in inflammation-induced apoptosis, together with a reductin in the synthesis of neurotrophic factors caused by a rise in brain glucocorticoids, may play a role in the pathology of these disorders. A reduction in the neuroprotective components of the kynurenine pathway, such as kynurenic acid, and an increase in the neurodegenerative components, 3-hydroxykynurenine and quinolinic acid, contribute to the pathological changes. Such changes are postulated t cause neuronal damage, and thereby predispose chronically depressed patients to demential.
Keywords: hypercortisolemia, inflammation, proinflammatory cytokine, prostaglandin E2, kynurenic acid, quinolinic acid, neurodegeneration
Abstract
Los estudios epidemiológicos demuestran que existe una correlación entre la depresión crónica y la probabilidad de desarrollar una demencia. Hay evidencias que los cambios inflamatorios en el cerebro constituyen características patológicas tanto de la depresión como de la demencia. Esto sugiere que un aumento en la apoptosis inducida por la inflamación, junto con una reducción en la síntesis de factores neurotróficos causada por un aumento en los glucocorticoides cerebrales, puede tener un papel en la patología de estos trastornos. Una reducción en los componentes neurodegenerativos, el ácido quinolínico y la 3-hidroxikinurenina, contribuyen a los cambios patológicos. Se postula que tales cambios causan daño neuronal y de esa manera predisponen a los pacientes con depresiones crónicas a la demencia.
Abstract
Des études épidémiologiques montrent qu'il existe une corrélation entre la dépression chronique et la probabilité d'une démence ultérieure. Il est prouvé que certaines modifications cérébrales de type inflammatoire sont des manifestations pathologiques à la fois de dépression et de démence. Ce qui suggère qu'une augmentation de l'apoptose provoquée par l'inflammation, accompagnée d'une réduction de la synthèse de facteurs neurotrophiques causée par une élévation des glucocorticoïdes cérébraux, puisse jouer un rôle dans la pathologie de ces troubles. Les modifications pathologiques sont dues à une réduction des composés neuroprotecteurs de la voie de la kynurénine, tels que l'acide kynurénique, et à une augmentation des composés neurodégénératifs, les acides 3-hydroxykynurénine et quinolinique. De tels changements sont pressentis comme étant la cause d'altérations neuronales, et prédisposent donc les déprimés chroniques à la démence.
Epidemiological studies have implicated chronic depression as an important predisposing factor for dementia in later life. Depression has been shown to be a common antecedent of Alzheimer's disease, and may be an early manifestation of dementia before the cognitive symptoms become apparent.1'2 In particular, patients with depression who later develop dementia usually have a poorer baseline performance in cognitive tasks.3
Several studies have shown that depression is a risk factor for dementia, particularly Alzheimer's disease, and this may be particularly important if the depressive episode occurs within 2 years of the diagnosis of demen? tia.3 Indeed, it has been estimated that patients with mild cognitive impairment and depression have more than twice the risk of developing dementia than those of the same age but who do not have depression. This suggests that depression may be a prodrome of dementia.4
Both depression and dementia are associated with inflammatory changes in the brain. The chronic inflammatory diseases, such as rheumatoid arthritis, are frequently associated with depression,5 while proinflammatory cytokines, such as interferon α (IFNα), used therapeutically in the treatment of hepatitis, for example, are known to precipitate depressive episodes in psychiatrically nondepressed patients.6 An experimental study has also been reported in which rats treated with IFNα showed anxiety behavior in open field, and changes in cytokines in both peripheral blood and in certain brain regions.7 Numerous clinical studies, supported by clinical evidence, have shown that proinflammatory cytokines are raised in the blood of depressed patients.8 Such observations form the basis for the macrophage theory of depression.9
The possible link between depression, dementia, and inflammatory changes in the brain is also supported by clinical and experimental studies of acquired immune deficiency syndrome (AIDS). It is well established that when human immunodeficiency virus (HIV)-infected patients develop AIDS, a substantial proportion of the patients also develop depression.10 Depression is one of the early man? ifestations of HIV dementia.11 Antiretroviral therapy was also one of those early manifestations.11 An experimental study in rodents showed that Efavirenz, the antiretroviral drug used in treatment of HIV infection, induced increased proinflammatory cytokines in the peripheral blood and was associated with anxiety behavior and impaired spatial memory.12 Thus, both depression and dementia are associated with inflammatory changes.
As there is pathological evidence that increased apoptosis occurs in both chronic depression and dementia, resulting in atrophic changes in the hippocampus, frontal cortex, and other brain regions,13-15 it has been speculated that the increase in inflammatory mediators, such as interleukin (IL)-1,TNFα, and prostaglandin E2 (PGE2), play a central role in the pathology of these conditions. The results of clinical and experimental research therefore lead to the conclusion that an increase in apoptosis caused by inflammation, together with a reduction in the synthesis of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) that assists in the repair of damaged neuronal networks, provide a basis for the pathological changes that are common to depression and dementia. The following reviews the evidence in favor of this hypothesis.
Changes in the hypothalamic-pituitary-adrenal axis in depression and dementia
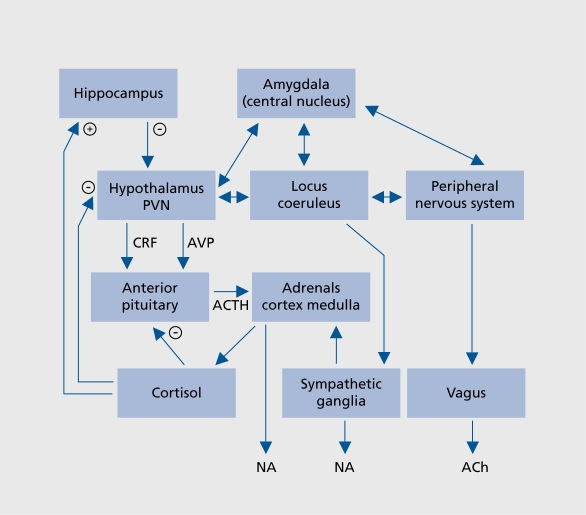
Stressful life events trigger neurotransmitter changes in the brain via activation of the corticotropin-releasing factor (CRF) pathway that terminates not only within the hypothalamus and other parts of the central endocrine system, but also on the locus ceruleus and raphe nuclei.16 This provides a biological link between stressful stimuli and the changes in the endocrine, immune, and neuro-transmitter systems that are involved in the psychopathology of depression (Figure 1.).
Figure 1. Relationship between stress, activation of limbic regions of the brain by CRF, and the consequent changes in the adrenal cortex and the sympathetic system. (+), activation, (-) inhibition. In chronic stress or depression, the feedback inhibitory loop malfunctions following the desensitization of the central glucocorticoid receptors in the brain and immune cells. This results in hypercortisolemia, a common feature of both major depression and Alzheimer's disease. Anxiety, a common comorbidity symptom with major depression, is associated with the increased activity of the central and peripheral sympathetic systems.17 CRF, corticotropin-releasing factor; NA, noradrenaline; AVP, arginine vasopressin; Ach, acetylcholine; ACTH, adrenocorticotropic hormone; PVN, paraventricular nucleus.
Investigations of the role of the hypothalamic-pituitaryadrenal (HPA) axis in the psychopathology of depression commenced over 40 years ago, when it was reported that depressed patients have a higher circulating plasma cortisol concentration than those that are not depressed.18,19 At this time, the dexamethasone depression test (DST) was developed to provide a functional assessment of HPA axis activity. It was discovered that this synthetic glucocorticoid would normally suppress the secretion of Cortisol by activating hypothalamic and pituitary glucocorticoid receptors, thereby suppressing the secretion of CRF and adrenocorticotropic hormone (ACTH) which, in turn, reduced the activation of the adrenal cortex and the release of Cortisol. The mechanism whereby these changes occurred was explained in terms of a negative feedback loop whereby the raised plasma glucocorticoid concentration controls the further release of the steroid. However, it soon became apparent that in patients with major depression the negative feedback loop ceased to function due to the desensitization of the central glucocorticoid receptors. The negative DST thereby became a diagnostic marker of melancholic depression.20
Nevertheless, it is now apparent that the DST lacks both specificity and sensitivity for depression,21 even though it may still offer reliability in the assessment of the severity of depression.22 Hypercortisolism and a negative DST are now known to occur in patients with Alzheimer's disease and alcoholism, for example.23 Furthermore, it has been estimated that only 60% of patients with major depression demonstrate a negative DST. Nevertheless, these findings do serve to emphasize the importance of the HPA axis in psychiatric disorders.
It is frequently assumed that the synthetic glucocorticoids such as dexamethasone act on glucocorticoid receptors in an identical manner to the natural glucocorticoids such as Cortisol. However, this may not be the case. Dexamethasone acts primarily on the glucocorticoid receptors in the anterior pituitary, does not readily enter the brain, and therefore differs substantially from natural glucocorticoids that activate both mineralocorticoid and glucocorticoid receptors.24 There is also evidence that, while dexamethasone may reduce the release of CRF, it does not suppress the release of arginine vasopressin (AVP). There is evidence that AVP, not CRF, is the main activator of the HPA axis due to chronic stress and major depression.25,26 The increased action of AVP is further exacerbated by the action of IL-1β; chronically administered IL-1β has been shown to cause a shift in the role of CRF to AVP in the activation of the anterior pituitary.27 In addition, it has been shown that there is an age-related increase in the colocalization of AVP in CRF neurons in patients with major depression and dementia.28 Thus, it seems reasonable to conclude that the hypersecretion of Cortisol in patients with depression or dementia may at least be partly a consequence of an increased activation of the HPA axis by AVP. Additional evidence for the change in the functional activity of the pituitary gland is provided by the finding that the adrenals and the pituitary are enlarged in those with depression,29,30 these changes being associated with a hypersecretion of CRF.31 Furthermore, the density of the CRF receptors in the frontal cortex are reduced, presumably as a consequence of the hypersecretion of CRF.32,33 The hypersecretion of CRF would appear to be a state, rather than a trait, marker of depression.34
If hypercortisolemia is a common feature of major depression and some types of dementia, it would be anticipated that immunosuppression would be a common feature of these conditions. However, it is apparent that both immunosuppression (for example, of natural killer cell [NKC] activity) and immune activation (for example, macrophage activation) are common features of depression. One possible explanation is that an increased vulnerability to environmental stress, which is a common feature of both depression and dementia,35 elicits a bidirectional, homeostatic interaction between the endocrine and immune systems. Thus, CRF has been associated with humoral activation that results in an increased release of proinflammatory cytokines. By activating the HPA axis, proinflammatory cytokines not only further release CRF, but also lead to glucocorticoid resistance, thereby impairing the regulatory feedback mechanism. Conversely, the increase in the concentration of plasma Cortisol, together with the increased sympathetic activity that is a normal feature of the stress response, suppresses NKC and T-cell replication. There is evidence that activation of the β-adrenoceptors on the NKC membrane, and which results in the decrease in activity of the NKCs, occurs independently of the activation of the HPA axis.35 Clearly the interaction between the immune system and the HPA axis is both complex and interdependent.
In the past 20 years, attention has focused on changes in the hypothalamic-pituitary-adrenal axis, together with the biogenic amine neurotransmitters noradrenaline, serotonin, and, to a lesser extent, dopamine.36,37 More recently, however, it has become apparent that both major depression and chronic stress result in more persistent structural changes in the brain as a consequence of the decrease in the synthesis of neurotrophic factors, such as BDNF and the antiapoptotic factor bcl-2.38 These changes are attributed to the chronic increase in brain glucocorticoids that arise due to the desensitization of central glucocorticoid type 2 receptors that occur as a consequence of the reduction in the inhibitory feedback mechanism.39 Such effects contribute to the failure in brain repair mechanisms which is indicated by a reduction in dendritic branching and a decrease in neurogenesis, particularly in the hippocampus and, to some extent, in the frontal cortex.40,41 Such changes, together with an activation of the proinflammatory cytokines by chronic stress and depression, also enhance apoptosis through their indirect excitotoxic and metabolic actions.42 Thus stress-induced hypercortisolemia and proinflammatory cytokines share a final common pathway that leads to impaired neuronal plasticity and deficits in central neurotransmission.
The possible link between hypercortisolemia and depression is further provided by the changes induced by antidepressants and glucocorticoid receptor antagonists such as mifepristone.43 Thus, preliminary clinical evidence has shown that the sensitization of the central glucocorticoid receptors by such treatments, that results in the re-establishment of the feedback inhibition of Cortisol release, are correlated with the attenuation of the symptoms of depression.44
Is there a link between depression and demential? The clinical perspective
There is overwhelming evidence that inflammatory changes are an important causative factor in the pathology of Alzheimer's disease and related dementias.45 The increase in β amyloid (Ab) is not only a major pathological feature of such dementias, but is also responsible for stimulating inflammatory responses in the brain. These changes include an increased expression of cell adhesion molecules and proinflammatory cytokines, and the activation of microglia in the brain parenchyma.46 In vitro studies have also demonstrated that Ab induces IL-lb and IFNg from vascular cells, thereby inducing a cascade of inflammatory changes.47,48 In addition, the infiltration of macrophages together with CD4+ and CD8+ T-cells, from the periphery have been detected in Ab deposits in cerebral vessels in patients with cerebral amyloid angiopathy.49
The combination of Ab and proinflammatory cytokines is linked to the increase in apoptosis in the brains of patients with dementia.50 For example, there is evidence that lymphocytes show a significant increase in DNA fragmentation in Alzheimer patients when compared with aged, but normal, controls.51 This change has been linked to an increase in the intracellular concentration of calcium ions, a prerequisite for apoptosis52 that has not been recorded in lymphocytes from aged control subjects. Furthermore, apoptotic cell death is preceded by the expression of apoptosis-associated molecules such as p53, Fas (CD95/APO-1) and IL-1b converting enzyme. Whereas the normal brain is partly immunologically privileged, in patients with inflammatory diseases such as multiple sclerosis, stroke, Alzheimer's disease, and possibly major depression, Fas is widely expressed in the brain.53 This apoptotic protein is expressed on CD4+ and CD8+ T-cells and on NKCs. Such observations provide a further link between the inflammatory changes in the brain and increased apoptosis that preludes dementia. Despite these convincing observations regarding the inflammatory changes in patients with Alzheimer's disease, it is somewhat surprising to find that IL-6, a major proinflammatory cytokine that is elevated in the plasma and cerebrospinal fluid (CSF) of patients with major depression, has been reported to be unchanged54 or even decreased55,56 in the blood of Alzheimer's patients. Some investigators have, however, reported that IL-6 is increased in these patients.57 Some of these differences may be accounted for by the methods used to assay IL-6. Thus the concentration of IL-6 in the serum and CSF is often at the limit of detection, while in invitro studies, in which stimulated lymphocytes are isolated by gradient centrifugation, the cells are stressed which may alter their phenotype.
It has also been argued that the decrease in proinflammatory cytokines in Alzheimer's disease is a consequence of the hypercortisolemia55 although this would not explain why cytokines such as IL-6 remain elevated in depressed patients where hypercortisolemia also commonly occurs.
The cognitive changes and dysphoria that are common symptoms in the early stages of Alzheimer's disease have been correlated with the increase in proinflammatory cytokines such as IFNα.6 Despite the equivocal evidence regarding the rise in plasma IL-6 concentration in Alzheimer patients, there are reports that the IL-6 concentration correlates with the severity of dementia.58 From the numerous studies of the changes in the immune system of patients with dementias, it would appear that the inflammatory changes can trigger an increased synthesis and accumulation of Ab.59 The accumulation of Ab then initiates a further cascade of inflammatory changes in the brain involving proinflammatory cytokines and neurotoxic free radicals such as nitric oxide (NO)60; this involves the activation of the NFkβ pathway and the complement system. Neuronal COX 2 expression is also increased in Alzheimer's disease, and the resulting increase in PGE2 contributes to the subsequent deterioration in the clinical state of the patient.61 In addition, the rise in IL-β may also indirectly contribute to the cognitive deficit by inhibiting cholinergic function62; a deficit in acetylcholine is generally accepted as the primary neurotransmitter that is causally involved in the cognitive and memory deficits in the dementias.44
The question arises as to whether the increase in Ab is a reflection of the rise in proinflammatory cytokines, an important consideration if major depression predisposes to dementia. In support of this connection, there is evidence that severe head trauma in young persons can result in a large number of amyloid plaques shortly after the traumatic event.63 The accumulation of Ab was shown to occur secondarily to the stress induced activation of the microglia that precipitate the release of Il-1 ; the Ab formed then stimulated the “cytokine cascade,” a key element in the pathogenesis of dementia.64
Further evidence in support of the hypothesis linking the outcome of chronic depression with dementia comes from studies on the progression of an HIV infection to AIDS. It is well known that severe life stress, and bereavement of a partner with AIDS, is associated with a rapid progression of HIV to AIDS and a consequent increase in mortality65 For example, it has been reported that changes in immune function, such as a reduction in NK cells, correlates with the incidence of depression and the progressive deterioration in the clinical status of the patients with HIV/ AIDS10,66,67 although not all investigators have found such an association.68 Nevertheless, such studies do provide possible support for the hypothesis that impaired immune function associated with the symptoms of depression may act not only in the progression of an AIDS infection but also to the onset of AIDS dementia in those patients who do not die as a consequence of secondary infections or cancer.
Changes in proinflammatory cytokines in depression and dementia
Evidence implicating a role for the proinflammatory cytokines in the etiology of depression has been provided by studies on the changes in IL-1, IL-6, and TNFα in depressed patients and also by the effects of IFNα on psychiatrically normal individuals being treated for hepatitis or a malignancy. Such studies have implicated these cytokines as causative factors in the symptoms of major depression. These symptoms include depressed mood, anxiety, cognitive impairment, lack of motivation, loss of libido, sleep disturbance, and deficits in short-term memory. Such symptoms usually disappear once the plasma cytokine concentrations return to normal.69 These changes appear to be a consequence of the neurotransmitter and endocrine changes induced by the cytokines, rather than the pathological condition for which the treatment has been administered.70,71 It is perhaps not surprising therefore to find that the symptoms of depression frequently occur in patients recovering from a chronic infection, those with multiple sclerosis,72 allergies,73 and rheumatoid arthritis.74 In all these situations, proinflammatory cytokines are known to be overexpressed75 The initial studies linking depression with an abnormality of the immune system,76 impaired mitogen-stimulated lymphocyte proliferation,77 and reduced NK cell activity78 in untreated depressed patients, showed changes that largely returned to normal once the patient recovered from the depressive episode. Recent research into the immune changes occurring in depression has concentrated on cytokines, soluble cytokine receptors, and plasma acute-phase proteins. For example, positive acute-phase proteins have been shown to increase while the negative acute-phase proteins decreased in depression, changes that are known to be a consequence of the action of IL-6 on liver function.79 In addition, complement proteins (C3,C4) and immunoglobulin M are increased in depressed patients. Such changes are evidence of immune activation involving both the inflammatory cytokines and B-cells that are activated by the proinflammatory cytokines. Further evidence of immune activation in depressed patients is provided by the studies showing that the plasma concentration of IL-1, IL-6, IFNg, soluble IL-6 and IL-2 receptors, and the IL-1 receptor antagonist, are raised. These changes are correlated with a rise in plasma acute-phase proteins.80 Effective antidepressant treatments largely attenuate such immune changes. In addition to the increases in proinflammatory cytokines, there is also evidence of an increased number of T-helper,T-memory, activated T-cells and B-cells that act as a source of the plasma cytokines.81-83 From these changes, it would appear that in depression there is an imbalance between the inflammatory and the anti-inflammatory arms of the immune system, the cytokines from the T1 pathway (such as IFNg) becoming predominant over those of the anti-inflammatory T2 (for example, IL-4) pathway. A recent study has shown that the T3 cytokine, transforming growth factor b1 (TGFβ1) whose function is to re-establish the balance between the T1 and T2 pathways, is increased in depressed patients following effective antidepressant treatment.84 Though TGFβ1 is reported as a regulatory cytokine that keeps the balance between Th1 and Th2 cytokines,85 precisely how the increases in the proinflammatory cytokines are attenuated by TGFβ1 in depressed patients is unclear.
The role of the microglia in inflammatory changes in the brain
Localized inflammatory responses in the brain parenchyma have been associated with the pathogenesis of a number of neurological disorders including Alzheimer's disease and Parkinson's disease.86,87 At these lesion sites, activated microglia release such inflammatory mediators as TNFα and PGE2.88 It is well-known that PGE2 is an important mediator of inflammation. In-vitro evidence shows that PGE2 secretion from lymphocytes of depressed patients is increased,89 as is the PGE2 content of the saliva, serum, and CSF of such patients.90,91 Of the proinflammatory cytokines, IL-6 appears to play a key role in the synthesis of this prostaglandin both in vitro and in vivo.92,93 Conversely, different types of antidepressants have been shown to inhibit the secretion of proinflammatory cytokines and to reduce the synthesis of PGE2.94,96 This raises the interesting possibility that the reduction in proinflammatory cytokines and inflammatory mediators such as PGE2 in the brain may be associated with the therapeutic actions of antidepressants.17 As it appears that the proinflammatory cytokines increase the inducible form of cyclo-oxygenase (COX2) in the brain, it would be expected that COX2 inhibitors would not only attenuate the central inflammatory changes but also exert an anti-depressant effect. There is some clinical evidence to support this view. Thus, rofecoxib, when administered to a large group of patients suffering from osteoarthritis, was found to reduce the symptoms of those who were suffering from comorbid depression; 15% of the patients had depression at the start of the study, which decreased to 3% at the end of the period of treatment.97 Other clinical studies have suggested that the COX2 inhibitor celecoxib has positive effects on cognitive function in depressed patients.97 It should be noted that celecoxib has also been shown to have beneficial effects as an “add-on” component to clozapine in the treatment of schizophrenia in patients who are only partially responding to the antipsychotic medication.98,99 There are several mechanisms that are postulated to be involved in the etiology of depression. It is commonly assumed that a decrease in both the noradrenergic and serotonergic functions are causally related to the changes in the mood, motivation, and cognitive changes associated with the disorder, There is now experimental evidence to show that the inhibition of COX2 is associated with a rise in the synthesis of serotonin in the cortex of the rat brain.100 In addition, PGE2 has been shown to reduce the release of noradrenaline from central noradrenergic neurons, an effect that would be blocked by the COX2 inhibitors. Thus inhibition of COX2 activity in the brain contributes not only to the reduction in inflammatory changes but also to an enhancement of biogenic amine function. PGE2 is probably one of the most potent inflammatory mediators in terms of the initiation and propagation of inflammation within the brain.101 Both clinical90,102 and experimental studies have shown that there is an increase in the tissue concentrations of PGE2 in depression and in an animal model of depression.89 In the brain, the microglia act as macrophages. On activation, they release proinflammatory cytokines, PGE2, and neurotoxic metabolites of the kynurenine pathway.103 Recent experimental evidence has shown that lipopoly saccharide (LPS), an activator of macrophage activity and a cause of brain inflammation, induces mitochondrial PGE2 synthase and COX2 activity in activated microglia, thereby increasing the synthesis of PGE2 at sites of inflammation in the brain.104 This provides a possible mechanism to explain the inflammatory changes in patients with depression or dementia; changes that contribute to neurodegeneration. Nitric oxide (NO) can also act as an inflammatory mediator that contributes to neurodegeneration,105 and is raised in the plasma of depressed patients.106 NO is produced by both the constitutive and inducible forms of NO synthase (NOS) that are associated with neurons and microglia.107-109
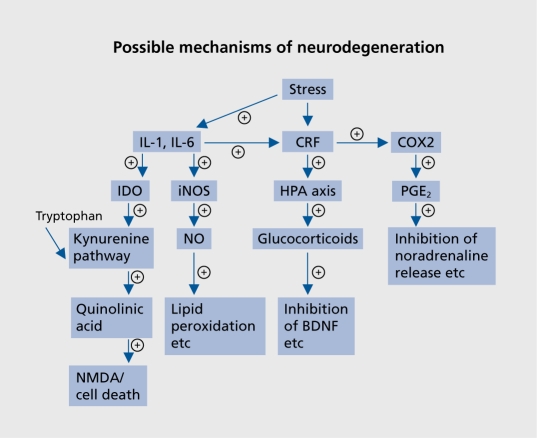
Recent evidence suggests that proinflammatory cytokines activate inducible NOS, thereby increasing NO; apoptosis results from the nitrosylation of deoxyribonucleic acid (DNA).110 The increase in peripheral and central macrophage activity associated with the inflammatory changes initiate, via the activated microglia, increases in PGE2 and NO that further potentiate the inflammatory changes (Figure 2.).Thus in both depression and dementia, PGE2, NO, and neurotoxic metabolites from the kynurenine pathway appear to play an important role central inflammatory processes that contribute to neurodegeneration.
Figure 2. Relationship between the main neurodegenerative pathways in the brain and depression. (+) Pathways that are increased in depression, and probably dementia.89,101,103,105,107 IDO, indoleamine 2,3-dioxygenase; iNOS, inducible nitric oxide synthase; CRF, corticotropin-releasing factor; COX 2, inducible cyclo-oxygenase 2; PGE2, prostaglandin E2; HPA, hypothalamicpituitary-adrenal; NO, nitrous oxide; IL, interleukin; NOS, nitrous oxide synthase; BDNF, brain-derived neurotrophic factor.
Neurodegeneration and the role of neurotoxic metabolites of the tryptophan pathway
The depletion of tryptophan from the diet results in a reduction in serotonin in the brain that correlates with the onset of a depressed mood state.111 Tryptophan is metabolized by two main pathways, by tryptophan hydroxylase leading to the synthesis of serotonin in the brain and by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygease (TDO) resulting in the formation of kynurenine.112,113 It has been hypothesized that in depression the metabolism of tryptophan by IDO and TDO is increased, thereby reducing the availability of the amino acid to synthesize serotonin.103 TDO is located in the liver, while IDO is found in the lungs, placenta, blood and brain.72,114 The activity of TDO is increased by tryptophan and by Cortisol. As hypercortisolemia frequently occurs in both depression and dementia, it would be anticipated that TDO is overactive in patients with these disorders. By contrast, IDO activity is increased by proinflammatory cytokines such as IL-6 and IFNg, and inhibited by anti-inflammatory cytokines such as IL-4.115,116
Thus the activities of both TDO and IDO are likely to be increased in depression and dementia as a consequence of the rise in circulating Cortisol and the proinflammatory cytokines. There are two main stages in the metabolism of tryptophan following the actions of the dioxygenases.117 Following the conversion of tryptophan to kynurenine by IDO or TDO, kynurenine is metabolized by kynurenine hydroxylase to the neurotoxic metabolites 3-hydroxykynurenine, 3-hydroxy-anthranilic acid, and quinolinic acid. An alternative pathway involves the conversion of kynurenine to 3-hydroxy anthranilic acid by kynureninase. These form the neurodegenerative arm of the tryptophan-kynurenine pathway.
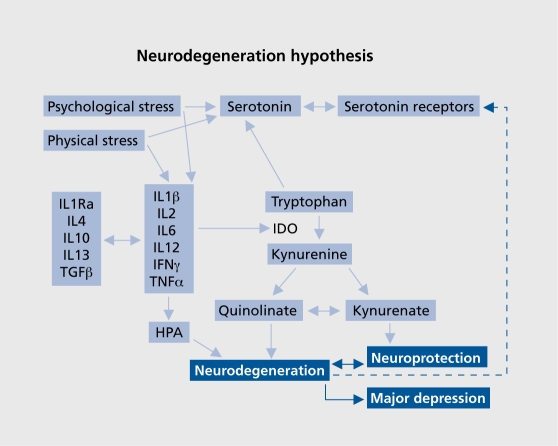
Alternatively, kynurenine may be metabolized by kynurenine aminotransferase to the neuroprotective end product kynurenic acid.118 The mechanisms whereby quinolinic and kynurenic acids act as neurotoxic and neuroprotective agents respectively is related to their activation or inhibition of the N-methyl-D-aspartate (NMDA) receptor, quinolinic acid and 3-hydroxyanthranilic acids being agonists of the NMDA receptor while kynurenic acid is an antagonist.119,120 It has also been hypothesized that the imbalance between those NMDA receptor antagonist and agonist are involved in the pathophysiology of chronic or treatment-resistant depression.104 In the brain, the metabolism of tryptophan by the enzymes of the kynurenine pathway occurs in both astrocytes and microglia121,122 the former producing mainly kynurenic acid while the latter produces the neurotoxic end products 3-hydroxy-kynurenine, 3-hydroxy anthranilic acid, and quinolinic acid.123 Astrocytes have been shown to metabolize quinolinic acid and thereby reduce the neurotoxic impact that may arise following microglia activation. From the foregoing evidence, it can be hypothesized that inflammatory changes in both depression and dementia involve the activation of microglia and an increase in the inflammatory challenge to the brain. Such changes also occur in patients with hepatitis who have been treated with the proinflammatory cytokine IFNα and who developed depressive symptoms as a side effect of the treatment. In these patients, it has been shown that the plasma kynurenic acid concentration was reduced, thereby suggesting that the neurodegenerative metabolites were increased.124 More recently we have shown that similar changes occur in the blood of patients with major depression.125 The results of this study also showed that therapeutically effective antidepressant treatment increased the neuroprotective kynurenic acid in the blood in those patients suffering from an acute episode of depression, but not in those with chronic depression. These changes occurred irrespective of the clinical improvement in the symptoms of the patients. This suggests that the progress to dementia may increase as the depression becomes more chronic. In patients with major depression, shrinkage of the hippocampus,126,127 a decrease in the number of astrocytes and a neuronal loss from the prefrontal cortex,41,128,129 and the striatum130 have been reported. Such findings support the view that neurodegenerative changes occur in several discrete regions of the brain in patients suffering from chronic depression. Furthermore, as the astrocytes are a major source of kynurenic acid, apoptosis of these cells would result in a reduction in the neuroprotective effect of kynurenic acid. There is evidence that in the astrocytes the kynurenine pathway is limited due to the absence of kynurenine hydroxylase. As a consequence, astrocytes only produce a very low concentration of the neurotoxin quinolinic acid and a relatively high concentration of the neuroprotective agent kynurenic acid.113 Furthermore, in astrocytes IDO is preferentially induced by IFNg, a cytokine that also induces the catabolism of quinolinic acid.113 However, it is also apparent that the increase in the synthesis of kynurenine by the astrocytes can indirectly contribute to the formation of quinolinic acid by the microglia. This situation would be compounded by the increased activation of the microglia by the proinflammatory cytokines with the consequent rise in the concentration of the inflammatory mediators PGE2 and NO. Figure 3. summarizes the pathways involved in the metabolism of tryptophan by the kynurenine pathway and the relationship with inflammatory cytokines in depression.
Figure 3. Outline of the kynurenine pathway, and its induction by proinflammatory cytokines, that results in the accumulation of the major neurotoxic metabolite, quinolinic acid.103,107,113 II, interleukin; TGF, transforming growth factor; IFN, interferon; IDO, indeolamine 2,3 dioxygenase; HPA, hypothalamic-pituitary-adrenal.
The inhibition of neuronal repair mechanisms resulting from the reduction in neurotrophic factors that follow the rise in blood and tissue Cortisol,131 apoptosis of astrocytes which are the sources of several neurotrophic factors,132 and the possible disruption of the phospholipase D pathway that has antiapoptotic properties and is involved in neurite formation and repair,133 further contribute to the neuronal loss. Another association between depression and dementia is through this IDO initiated kynurenine pathway related neurotoxicity. An immunohistochemical study has proven that the immunoreactivity of IDO and quinolinic acid are high in the hippocampus of Alzheimer's disease patients.134
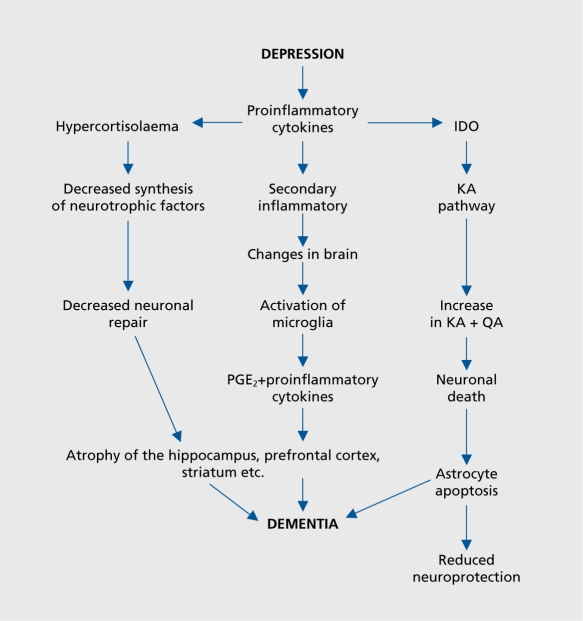
So far, emphasis has been placed on the role of inflammatory mediators and neurotoxins produced by the kynurenine pathway on the possible causes of the neurodegenerative changes in the brain that eventually develops into dementia. Recently, experimental evidence has shown that transgenic mice that overexpress human tau protein (a prominent feature of different types of dementia) show depressive-like behavior in the Forced Swim Test. This test is widely used to predict antidepressant activity, and is based on the observation that when rodents are placed in a container of warm water from which they cannot escape, they soon adopt an immobile posture. This is assumed to reflect a state of “learned helplessness” that reflects a depressive-like state.135 This behavioral state was reversed by the administration of the selective serotonin reuptake inhibitor antidepressant fluvoxamine. In-vivo microdialysis studies showed that the release of serotonin from the prefrontal cortex was reduced in the transgenic mice, an effect that was reversed by the fluvoxamine treatment. The results of this study suggest that transgenic mice overex-pressing human tau protein show symptoms of depressivelike behavior that are associated with a reduction in serotonergic function. As the behavioral and neurotransmitter changes are reversed by a selective serotonin reuptake inhibitor (SSRI) antidepressant, it would appear that serotonin may provide a link between the pathological effects of tau protein and the subsequent depressive-like state. It would be incautious to extrapolate from this subchronic study in a transgenic mouse to the complex clinical situation in which multiple pathological changes contribute to the onset of dementia. Nevertheless, the experimental studies do provide evidence in support of the hypothesis that the long-term outcome of chronic depression is often dementia. Further evidence for this hypothesis comes from the study by Steffens et al4 who demonstrated a link between late-onset depression and the rise in plasma apolipoprotein E4 which is widely considered to be a risk factor for late-onset Alzheimer's disease. Figure 4. summarizes the possible pathways leading from depression to dementia.
Figure 4. Theoretical pathway linking chronic depression to dementia. PGE2, prostaglandin E2; ID0, indeolamine 2,3 dioxygenase; KA, kynurenic acid; QA, quinolinic acid.
Conclusion
Neuronal loss is a common feature of major depression and dementia. The progress of major depression to dementia could result from the chronic inflammatory changes that are linked to the activation of the microglia. The activation of inducible COX2 and NOS by the proinflammatory cytokines further increases the inflammatory challenge to the brain. As there is evidence that the kynurenine pathway is also activated by proinflammatory cytokines, it seems likely that the concentrations of the neurotoxins 3-hydroxy kynurenine, 3-hydroxyanthranillic acids, and quinolinic acid will also increase as a result of the activation of the microglia. The increased apoptosis of the astrocytes, with a reduction in the availability of the neuroprotective agent kynurenic acid, further adds to the impact of the neurodegenerative changes. Hypercortisolemia, a common feature of both dementia and major depression, and apoptosis of astrocytes decreases the synthesis of neurotrophic factors thereby reducing neuronal repair. This process may be further enhanced by the disruption of the phospholipase D pathway that normally plays an important role in neurite formation and neuronal repair. This hypothesis may assist in explaining the degenerative changes in the hippocampus and other brain regions that are the features of chronic major depression. It may also explain why chronic depression is frequently a prelude to dementia in the elderly patient.
Selected abbreviations and acronyms
- AIDS
acquired immune deficiency syndrome
- BDNF
brain-derived neurotropic factor
- COX2
cyclo-oxygenase
- CRF
corticotropin-releasing factor
- HPA axis
hypothalamic-pituitary-adrenal axis
- IFN
interferon
- IL
interleukin
- NO
nitrous oxide
- PGE2
prostaglandin E2
- TGF
transforming growth factor
A. M. Myint thanks the Universities of Maastricht and Antwerp for their financial support that enabled her to undertake the research that forms part of this presentation.
Contributor Information
Brian E. Leonard, Author affiliations: Department of Pharmacology, National University of Ireland, Galway, Ireland.
Ayemu Myint, Brain and Behaviour Research Institute, Department of Psychiatry and Neuropsychology, University of Maastricht, the Netherlands.
REFERENCES
- 1.Geerlings Ml., Schoevers RA., Beckman AT., et al. Depression and risk of cognitive decline and Alzheimer's disease. Results of two prospective community based studies in the Netherlands. Br J Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 2.Visser PJ., Verhey FR., Ponds RW., et al. Distinction between preclinical Alzheimer's disease and depression. J Am Geriatr Soc. 2000;48:479–484. doi: 10.1111/j.1532-5415.2000.tb04992.x. [DOI] [PubMed] [Google Scholar]
- 3.Modrego PJ., Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of the Alzheimer type: a prospective study. Arch Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 4.Steffens DC., Plassman BL., Helms MJ., et al. A twin study of late-onset depression and apolipoprotein E4 as risk factors for Alzheimer's disease. Biol Psychiatry. 1997;41:851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- 5.Campbell IK., Roberts LJ., Wicks IP. Molecular targets in immune mediated diseases: the case of TNF and rheumatoid arthritis. Immunol Cell Biol. 2003;81:354–366. doi: 10.1046/j.0818-9641.2003.01185.x. [DOI] [PubMed] [Google Scholar]
- 6.Licinio J., Kling MA., Hauser P. Cytokine and brain function: relevance to IFNalpha induced'mood and cognitive changes. Sem Oncol. 1998;25:30–38. [PubMed] [Google Scholar]
- 7.Myint A-M., O'Mahony SM., Kubera M., et al. Role of paroxetine in interferon- induced immune and behavioural changes in male Wistar rats. J Psychopharmacol. 2006. In press. doi: 10.1177/0269881107077165. [DOI] [PubMed] [Google Scholar]
- 8.Maes M., Bosnians E., De Jongh R., et al. Increased serum IL6 and IL1-R antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 9.Smith R. The macrophage theory of depression. Med Hypoth. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 10.Mayne TJ., Vittinghoff E., Chesney MA., et al. Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Med. 1996;156:223–2238. [PubMed] [Google Scholar]
- 11.Stern Y., McDermott MP., Albert S., et al. Factors associated with incident human immunodeficiency virus-dementia. Arch Neurol. 2001;58:473–479. doi: 10.1001/archneur.58.3.473. [DOI] [PubMed] [Google Scholar]
- 12.O'Mahony SM., Myint A-M., Steinbusch H., Leonard BE. Efavirenz induces depressive like behaviour, increased stress response and changes in the immune response in rats. Neuroimmunomodulation. 2005;12:293–298. doi: 10.1159/000087107. [DOI] [PubMed] [Google Scholar]
- 13.Gilbertson HW., Sherton ME., Ciszewski A., et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature. Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen BS. Possible mechanisms for atrophied human hippocampus. Mol Psychiatry. 1997;2:255–262. doi: 10.1038/sj.mp.4000254. [DOI] [PubMed] [Google Scholar]
- 15.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 16.Gold P., Chrousos G., Kellner C., et al. Psychiatric implications of basic and clinical studies with. CSt. Am J Psychiatry. 1984;141:619–627. doi: 10.1176/ajp.141.5.619. [DOI] [PubMed] [Google Scholar]
- 17.Leonard BE. Brain cytokines and the psychopathology of depression. In: Leonard BE, ed. Antidepressants. Basel, Switzerland: Birkhauser Verlag. 2001:109–120. [Google Scholar]
- 18.Board F., Wadeson R., Persky H. Depressive affect and endocrine function: blood levels of adrenal cortex and thyroid hormones in patients suffering from depressive reactions. Arch Neurol Psychiatry. 1957;78:612–620. [PubMed] [Google Scholar]
- 19.Sachar E., Hellman L., Fukushima D., Gallagher T. Cortisol production in depressive illness. Arch Gen Psychiatry. 1970;23:289–298. doi: 10.1001/archpsyc.1970.01750040001001. [DOI] [PubMed] [Google Scholar]
- 20.Carroll B. Pituitary-adrenal function in depression. Lancet. 1968;556:1373–1374. doi: 10.1016/s0140-6736(68)92072-2. [DOI] [PubMed] [Google Scholar]
- 21.Arana G., Mossman D. The DST and depression: approaches to the use of a laboratory test in psychiatry. Neurol Clin. 1988;6:21–39. [PubMed] [Google Scholar]
- 22.Trapp T., Holsboer F. Heterodimerzation between mineralocorticoid and glucocorticoid receptors increase the functional diversity of corticosteroid action. Trends Pharmacol Sci. 1996;17:145–149. doi: 10.1016/0165-6147(96)81590-2. [DOI] [PubMed] [Google Scholar]
- 23.Evans D., Nemeroff C. Use of the DST using DSM 3 criteria on an inpatient psychiatric unit. Biol Psychiatry. 1983;18:505–511. [PubMed] [Google Scholar]
- 24.Hartmann A., Veldhuis JD., Deuschle M., et al. 24 hour Cortisol release profiles in patients with Alzheimer's disease and Parkinbson's disease compared to normal controls:ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18:285–289. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 25.Davis KL., Mohs RC., Marin DB., et al. Neuropeptide abnormalities in cortical depletion paralleled by increase in CRF receptors and general functional regulation of the CRF/HPA axis. Arcs Gen Psychiatry. 1999;56:981–987. doi: 10.1001/archpsyc.56.11.981. [DOI] [PubMed] [Google Scholar]
- 26.Scott LV., Dinan TG. Vasopressin and the regulation of the HPA axis function: implications for the pathophysiology of depression. Life Sci. 1998;62:1985–1998. doi: 10.1016/s0024-3205(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 27.Bartanusz J., Jezova D., Bertinï LT., et al. Stress-induced increases in vasopressin and CRF expression in hypophysiotrophic paraventricular neurons. Endocrinology. 1993;132:895–902. doi: 10.1210/endo.132.2.8425502. [DOI] [PubMed] [Google Scholar]
- 28.Raadsheer FC., vanHeerikhuïzen J., Lucassen PJ., et al. Increased CRHmRNA in paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 29.Amsterdam J., Marinelli D., Arger P., Winokur A. Assessment of adrenal gland volume by computed tomography in depressed patients and healthy volunteers. 1987;21:189–197. doi: 10.1016/0165-1781(87)90022-9. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan KR., McDonald WM., Escalona PR., et al. Magnetic resonance imaging of the caudate nuclei in depression: preliminary observations. Arch Gen Psychiatry. 1992;49:553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 31.Nemeroff C., Wïderov E., Bïssette G., et al. Elevated concentration of CRFlike immunoreactïvïty in CSF of depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 32.Hucks D., Lowther S., Crompton M., et al. CSF binding sites in cortex of depressed suicides. Psychopharmacol. 1997;134:174–178. doi: 10.1007/s002130050439. [DOI] [PubMed] [Google Scholar]
- 33.Nemeroff C., Owens M., Bïssette G., Andorn A. Reduced CRF binding sites in frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 34.Newport DJ., Nemeroff C. HPA axis: normal physiology and disturbances in depression. In: Thakore J, ed. Physical Consequences of Depression. Petersfield, UK: Wrightson Biomed Publishing Ltd. 2001:1–22. [Google Scholar]
- 35.Irwin M. Stress-induced immune suppression:the role of CRF and ANS mechanisms. Adv Neuroimmunol. 1994;4:29–47. doi: 10.1016/s0960-5428(06)80188-9. [DOI] [PubMed] [Google Scholar]
- 36.Karten YJ., Nair SM., van Essen L., et al. Long term exposure to high corticosterone levels attenuates serotonin responses in rat hïppocampal CA1 neurons. Proc Nat Acad Sci U S A. 1999;96:13456–13461. doi: 10.1073/pnas.96.23.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linthorst AC., Fladiskamm C., Hopkins SJ., et al. Long-term intracerebroventricular infusion of CRF alters neuroendocrine, neurochemical, autonomic, behavioural and cytokine responses to a systemic inflammatory challenge. J Neurosci. 1997; 17:4448–4460. doi: 10.1523/JNEUROSCI.17-11-04448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duman RS., Henninger GR., Nessler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 39.Sapolsky RM. Glucocorticoids and hïppocampal atrophy in neuropsychiatrie disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 40.Bremner JD., Narayan M., Anderson ER., et al. Hïppocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 41.Ongur D., Drevets WC., Price JL. Glia reduction in subgenual pre-frontal cortex in mood disorders. Proc Nat Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayley S., Poulter MO., Merali Z., Anïsman H. The pathogenesis of clinical depression: stressor and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 43.Belanoff JK., Rothschild AJ., Cassidy F., et al. An open label trial of mifepristone for psychotic major depression. Biol Psychiatry. 2002;52:386–392. doi: 10.1016/s0006-3223(02)01432-4. [DOI] [PubMed] [Google Scholar]
- 44.Dringenberg HC. Alzheimer's disease: more than a cholinergic disorderevidence that cholinergic-monoamineergic interactions contribute to EEG slowing and dementia. Brain Behav Res. 2000;115:235–249. doi: 10.1016/s0166-4328(00)00261-8. [DOI] [PubMed] [Google Scholar]
- 45.McGeer PL., McGeer EG. The inflammatory response system in the brain: implications for therapy of Alzheimer's disease and other neurodegenerative diseases. Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 46.Eilelenbloome P., Veerhuis R. The role of complement and activated microglia in the pathogenesis of Alzheimer's disease. Neurobiol Aging. 1996;28:83–988. doi: 10.1016/0197-4580(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 47.Raitano AB., Korc M. TNF upregulates IFN gamma binding in a human carcinoid cell. line. J Biol Chem. 1990;265:10466–10472. [PubMed] [Google Scholar]
- 48.Suo Z., Tan J., Placzek A., et al. Alzheimer's beta amyloid peptides induce an inflammatory cascade in human vascular celkthe roles of cytokines and CD40. Brain Res. 1998;807:110–117. doi: 10.1016/s0006-8993(98)00780-x. [DOI] [PubMed] [Google Scholar]
- 49.Kendler K., Karkowski L., Prescott C. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 50.McGeer PL., Rogers J., McGeer E. Neuroimmune mechanisms in Alzheimer's disease pathogenesis. Aiz Dis Assoc Discord. 1994;8:149–158. doi: 10.1097/00002093-199408030-00001. [DOI] [PubMed] [Google Scholar]
- 51.Eckert A., Cotman CW., Zerfass R., et al. Lymphocytes as a cell model to studyapoptosois in Alzheimer's disease: vulnerability to programmed cell death appears to be altered. J Neural Trans. 1998;54 (suppl 4): 259–267. doi: 10.1007/978-3-7091-7508-8_25. [DOI] [PubMed] [Google Scholar]
- 52.Sulgar J., Dumais-Huber C., Zerfass R., et al. The calcium response of human T-lymphocytes is decreased in aging but increased in Alzheimer's dementia. Biol Psychiatry. 1999;45:737–742. doi: 10.1016/s0006-3223(98)00218-2. [DOI] [PubMed] [Google Scholar]
- 53.Nishïmura T., Akïyama H., Yonehara S., et al. Fas antigen expression in brains of patients with Alzheimer's type dementia. Brain Res. 1995;695:137–145. doi: 10.1016/0006-8993(95)00699-q. [DOI] [PubMed] [Google Scholar]
- 54.Schott K., Richartz E., Noda S., et al. Immunological alterations in Alzheimer's disease. In: Sperner-Unterweger B, Fleischhaker WW, Kaschka UP, eds. Psychoneuroimmunology: Hypothesis and Current Research. Basel, Switzerland: Karger. 2001:120–126. [Google Scholar]
- 55.Yamada M. Immune reactions associated with cerebral amyloid angiopathy. Stroke. 1995;27:1155–1162. doi: 10.1161/01.str.27.7.1155. [DOI] [PubMed] [Google Scholar]
- 56.Maerz P., Heese K., Hoch C., et al. IL-6 and SiI-6R are not altered in the CSF of Alzheimer's disease patients. Neurosci Lett. 1997;239:29–32. doi: 10.1016/s0304-3940(97)00886-0. [DOI] [PubMed] [Google Scholar]
- 57.Luterman JD., Harontunian V., Yemal S., et al. Cytokine gene expression as a function of the clinical progression of Alzheimer's dementia. Arch Neurol. 2000;57:1153–1160. doi: 10.1001/archneur.57.8.1153. [DOI] [PubMed] [Google Scholar]
- 58.Blum-Degen D., Mueller T., Kuhn W., et al. IL-1 beta and IL-6 elevated in the CSF of Alzheimer's disease and de novo Parkinson's disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 59.Kalman J., Juhasz A., Laird C., et al. Serum IL-6 levels correlate with the severity of dementia in Downs syndrome and Alzheimer's disease. Acta Neurol Scand. 1997;96:236–240. doi: 10.1111/j.1600-0404.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 60.Hu J., Akama KT., Krafft GA., et al. Amyloid beta peptide activates cultured astrocytes: morphological alterations,cytokine induction and nitric oxide release. Brain Res. 1998;785:195–206. doi: 10.1016/s0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 61.Roberts GW., Gentleman SM., Lynch A., Graham Dl. Beta A4 amyloid protein deposition in the brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 62.Solomon GF., Morley JE. Psychoneuroimmunology and aging. In: Ader R, Felton DL, Cohen N, eds. Psychoneuroimmunology. Vol 2. 3rd ed. New York, NY: Academic Press. 2001:701–717. [Google Scholar]
- 63.Rada P., Mark GP., Vitek MP., et al. IL-1 beta decreases acetylcholine measured by microdialysis in hippocampus of freely moving rats. Brain Res. 1991;550:287–290. doi: 10.1016/0006-8993(91)91330-4. [DOI] [PubMed] [Google Scholar]
- 64.Ho L., Purohit D., Haroutunian V., et al. Neuronal COX 2 expression in the hippocampus on function and clinical progression of Alzheimer's disease. Arch Neurol. 2001;58:487–492. doi: 10.1001/archneur.58.3.487. [DOI] [PubMed] [Google Scholar]
- 65.Evans DL., Leserman J., Perkins DO., et al. Severe life stress as a predictor of early disease progression in HIV infection. Am J Psychiatry. 1997;154:630–634. doi: 10.1176/ajp.154.5.630. [DOI] [PubMed] [Google Scholar]
- 66.Lyketon CG., Hoover DR., Guccione M. Depression and survival among HIV infected persons. JAMA. 1996;275:35–36. doi: 10.1001/jama.1996.03530250039021. [DOI] [PubMed] [Google Scholar]
- 67.Rabkin JG., Williams JBW., Remien RH., et al. Depression distress, lymphocyte subsets in HIV positive symptoms on two occasions in HIV positive homosexual. men. Arch Gen Psychiatry. 1991;48:111–119. doi: 10.1001/archpsyc.1991.01810260019002. [DOI] [PubMed] [Google Scholar]
- 68.Gallant JE. Initial therapy of HIV infection. J Clin Virol. 2002;25:317–333. doi: 10.1016/s1386-6532(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 69.Meyers CA., Valentine AD. Neurologic and psychiatric adverse effects of immunological therapy. CNS Drugs. 1995;3:56–68. [Google Scholar]
- 70.Hansen MK., Taish P., Chen Z., Kreuger JM. Vagotomy blocks the induction of IL-1 beta. J Neurosci. 1998;18:2247–2253. doi: 10.1523/JNEUROSCI.18-06-02247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mellor AL., Munn DH. Tryptophan catabolism and T-cell tolerance:immunosuppression by starvation. Immunol Today. 1999;20:69–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 72.Banks WA., Kastin AJ., Ehrensing CA. Blood-borne IL-1 alpha is transported across endothelial blood-spinal cord barrier in mice. J Physiol. 1994;479:257–264. doi: 10.1113/jphysiol.1994.sp020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marshall PS. Allergy and depressions a neurochemical threshold model of the relation between the illnesses. Psychol Bull. 1993;113:23–43. doi: 10.1037/0033-2909.113.1.23. [DOI] [PubMed] [Google Scholar]
- 74.Katon W., Sullivan MD. Depression and chronic medical illness. J Clin Psychiatry. 1990;51:3–11. [PubMed] [Google Scholar]
- 75.Schrott LM., Crnic LS. Anxiety behaviour, exploratory behaviour and activity in NZB x and NZB F1 hybrid mice: role of b genotype and autoimmune disease progression. Brain Behav Immun. 1996;10:260–274. doi: 10.1006/brbi.1996.0023. [DOI] [PubMed] [Google Scholar]
- 76.O'Neill B., Leonard BE. Is there an abnormality in neutrophil phagocytosis in depression? IPCS Med Sci. 1986;14:802–803. [Google Scholar]
- 77.Kronfol Z., House JD. Depression, HPA activity and lymphocyte function. Acta Psychiat Scand. 1987;80:142–147. [Google Scholar]
- 78.Irwin M., Smith TL., Gillin JC. Low natural killer cell cytotoxicity in major depression. Life Sci. 1987;41:2127–2133. doi: 10.1016/0024-3205(87)90531-5. [DOI] [PubMed] [Google Scholar]
- 79.Song C., Dinan T., Leonard BE. Changes in immunoglobulins, complement and acute phase bproteins in depressed patients and normal controls. J Affect Dis. 1994;30:283–288. doi: 10.1016/0165-0327(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 80.Sluzewska A., Rybakowski J., Bosnians E., et al. Indicators of immune activation in major depression. Psychiatry Res. 1996;64:162–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 81.Neveu PJ., Castanon N. Is there evidence for an effect of antidepressant drugs on immune function? In: Dantzer R, Wollman EE, Yirmiya R, eds. Cytokines, Stress and Depression. New York, NY: Kluwer Academic/Plenum Press. 1999:267–281. doi: 10.1007/978-0-585-37970-8_15. [DOI] [PubMed] [Google Scholar]
- 82.O'Connor TJ., Harkin A., Kelly JP., Leonard BE. Olfactory bulbectomy provokes suppression of IL-1 beta and TNF alpha production in response to an in vivo challenge with LPS: effect of chronic desipramine treatment. Neuroimmunomodulation. 2000;7:27–31. doi: 10.1159/000026417. [DOI] [PubMed] [Google Scholar]
- 83.Xia Z., de Pierre JW., Nassberger L. Tricyclic antidepressants inhibit IL-6JL1 beta,and TNF alpha release in human blood monocytes and IL-2 and interferon in T-cells. Immunopharmacol. 1996;34:27–37. doi: 10.1016/0162-3109(96)00111-7. [DOI] [PubMed] [Google Scholar]
- 84.Myint A-M., Leonard BE., Steinbusch HW., Kim Y-K. Th1, Th2 and Th3 cytokine alterations in major depression. J Affect Dis. 2005;88:169–173. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Prud'homme GJ., Piccirillo CA. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. J Autoimmun. 2000;14:23–42. doi: 10.1006/jaut.1999.0339. [DOI] [PubMed] [Google Scholar]
- 86.Aisen PS. Inflammation and Alzheimer's disease. Mol Chem Neuropatho. 1996;28:83–88. doi: 10.1007/BF02815208. [DOI] [PubMed] [Google Scholar]
- 87.McGeer PL., Itagaki S., Boyes BE., McGeer EG. Reactive microglia are positive for HLA-DR in the sustantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1998;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 88.Liu B., Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases:mechanisms and strategies for therapeutic interventions. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 89.Song C., Lin A., Bonaccorso S., et al. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J. Affect Dis. 1998;49:211–219. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 90.Linnoila M., Whorton R., Rubinow DR., et al. CSF prostaglandin levels in depressed and schizophrenic patients. Arch Gen Psychiatry. 1983;40:405–406. doi: 10.1001/archpsyc.1983.01790040059008. [DOI] [PubMed] [Google Scholar]
- 91.Ohishï K., Ueno R., Nishïno S., et al. Increased level of salivary prostaglandins in patients with major depression. Biol Psychiatry. 1988;23:326–334. doi: 10.1016/0006-3223(88)90283-1. [DOI] [PubMed] [Google Scholar]
- 92.Akarasereen P., Techatriscik K., Chottewuttaken S., et al. The induction of COX 2 in IL-1 beta treated endothelial cells is inhibited by PGE2 through cAMP. Médiat Infiamm. 1999;8:287–294. [Google Scholar]
- 93.Schmidlïn F., Loeffler S., Bertrand C., et al. PLA2 phosphorylation and cyclooxygenase 2 induction, through p38 MAP kinase pathway, is involved in the IL-1 beta-induced bradykïnïn B2 receptor gene transcription. N S Arch Pharmacol. 2000;361:247–254. doi: 10.1007/s002109900191. [DOI] [PubMed] [Google Scholar]
- 94.Leonard BE., Song C. Stress, Depression and the role of cytokines. In: Dantzer R, Wollman EE, Yîrmïya R, eds. Cytokines, Stress and Depression. New York, NY: Kluwer Academic/Plenum Press. 1999:251–265. [Google Scholar]
- 95.Mtabaji JP., Manku MS., Horrobin DS. Actions of the tricyclic antidepressant clomipramine on responses to pressor agents interactions with PGE2. Prostaglandins. 1977;14:125–132. doi: 10.1016/0090-6980(77)90161-7. [DOI] [PubMed] [Google Scholar]
- 96.Glen AIM., Ross BM. Prostaglandins and eicosanoids in mental illness. In: Curtis-Prior P, ed. 777 e. Eicosanoids. Chichester, UK: John Wiley and Sons. 2004:493–498. [Google Scholar]
- 97.Collantes-Esteres E., Fernandez-Perrez C. Impaired self control of osteoarthritis pain and self reported health states in non-responders to celecoxib switched to rofecoxib: results of PAVIA, an open label post marketing survey in Spain. Curr Med Res Opin. 2003;19:402–410. doi: 10.1185/030079903125001938. [DOI] [PubMed] [Google Scholar]
- 98.Mueller N., Riedel M., Schwarz MJ., Engel RR. Clinical effects of COX2 inhibitors on cognition in schizophrenia. Eur Arch Psychiat Clin Neurosci. 2004;254:149–151. doi: 10.1007/s00406-004-0548-4. [DOI] [PubMed] [Google Scholar]
- 99.Mueller N., Strassnig M., Schwarz MJ., et al. COX2 inhibitors as adjunctive therapy in schizophrenia. Exp Opinion Invest Drugs. 2004;13:1033–1044. doi: 10.1517/13543784.13.8.1033. [DOI] [PubMed] [Google Scholar]
- 100.Sandrini M., Vitale G., Pini LA. Effect of rofecoxib on nociception and the serotonin system in the rat brain. Infiamm Res. 2002;51:154–159. doi: 10.1007/pl00000287. [DOI] [PubMed] [Google Scholar]
- 101.Griffin DE., Wesselingh SL., McArthur JC. Elevated CNS prostaglandins in human immunodeficiency virus associated dementia. Ann Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- 102.Calabrese JR., Skwerer AG., Barna B., et al. Depression, immunocompetence and prostaglandins of the E series. Psychiat Res. 1986;17:41–47. doi: 10.1016/0165-1781(86)90040-5. [DOI] [PubMed] [Google Scholar]
- 103.Myint A-M., Kim Y-K. Cytokine-serotonin interactions through indoleamine 2,3-dixygenase: a neurodegenerative hypothesis of depression. MedHypoth. 2003;61:519–525. doi: 10.1016/s0306-9877(03)00207-x. [DOI] [PubMed] [Google Scholar]
- 104.Ikeda-Matsuo Y., Ikegaya Y., Matsuki N., et al. Microglia specific expression of microsomal prostaglandin E2 synthase-1 contributes to lipopolysaccharide-induced PGE2 production. J Neurochem. 2005;94:1546–1558. doi: 10.1111/j.1471-4159.2005.03302.x. [DOI] [PubMed] [Google Scholar]
- 105.Dawson VL., Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 106.Suzuki E., Yagï G., Nakakï T., et al. Elevated plasma nitrate levels in depressive states. J Affect Dis. 2001;63:221–224. doi: 10.1016/s0165-0327(00)00164-6. [DOI] [PubMed] [Google Scholar]
- 107.Harvey BH. Affective disorders and nitric oxide: a role in pathways to relapse and refractoriness? Hum. Psychopharmacol. 1996;11:309–319. [Google Scholar]
- 108.Moncada S., Higgs A., Furchgott R. International Union of Pharmacology: nomenclature in nitric oxide research. Pharmacol Rev. 1997;49:137–142. [PubMed] [Google Scholar]
- 109.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 110.Cuzzocrea S., Riley DP., Caputi AP., et al. Antioxidant therapy: a new approach in shockïnflammatïon and ischaemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 111.Young SN., Smith PE., Piht RO., Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacol (Bert) 1985;87:173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 112.Guillemin GJ., Kerr SJ., Smythe GA., et al. The kynurenine pathway metabolism in human astrocytes: a pathway for neuroprotection. J Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 113.Hayaishi O. Biochemical and Medical Aspects of Tryptophan Metabolism. Amsterdam: Elsevier/North Holland Biomedical Press; 1980. [Google Scholar]
- 114.Heyes MP., Saito K., Major EO., et al. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Brain. 1993;16:1425–1450. doi: 10.1093/brain/116.6.1425. [DOI] [PubMed] [Google Scholar]
- 115.Musso T., Gusella GL., Brooks A., et al. IL-4 inhibits indoleamine dioxygenase expression in human monocytes. Blood. 1994;83:1408–1411. [PubMed] [Google Scholar]
- 116.Carline JM., Borden EC., Sondel PM., Byrne GL. Biologic response modifier induced indoleamine 2,3-dioxygenase activity inhuman peripheral blood mononuclearcell cultures. J Immunol. 1987;139:2414–2418. [PubMed] [Google Scholar]
- 117.Chiarugï A., Calvani M., Meli E., et al. synthesis and release of neurotoxic kynurenine metabolites by human derived macrophages. J Neuroimmunol. 2001;120:190–198. doi: 10.1016/s0165-5728(01)00418-0. [DOI] [PubMed] [Google Scholar]
- 118.Perkins MN., Stone TW. An iontophorectic investigation of the actions of convulsant kynurenines and their interaction with endogenous quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 119.Kim JP., Choi DW. Quinolinate neurotoxicity in cortical cell culture. Neuroscience. 1987;23:423–432. doi: 10.1016/0306-4522(87)90066-2. [DOI] [PubMed] [Google Scholar]
- 120.Stone TW., Darlington LG. Endogenous kynureninsas as targets for drug discovery and development. Nat Rev Drug Discovery. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 121.Grant RS., Kapoor V. Murine glial cells regenerate NAD, after peroxideinduced depletion,using either nicotinic acid, nicotinamide or quinolinic acid as substrates. J Neurochem. 1998;70:1759–1763. doi: 10.1046/j.1471-4159.1998.70041759.x. [DOI] [PubMed] [Google Scholar]
- 122.Heyes MP., Achim CL., Wiley CA., et al. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1996;320 (part 2):595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guillemin GJ., Smythe G., Takikawa O., Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 124.Wïchers MC., Koek GH., Robaeys G., et al. IDO and interferon alpha induced depressive symptoms: a shift in hypothesis from tryptophan depletion. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 125.Myint AM., Kim Y-K., Vrkerk R., et al. Tryptophan metabolites in major depression:evidence of an inbalance in neurodegeneration and neuroprotection. J Affect Dis. 2006. In press. [Google Scholar]
- 126.Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 127.Sheline Yl., Sanghavi M., Mintun Gado MH. Depression duration, but not age, predicts hïppocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cotter DR., Pariante CE., Everall IP. Gial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 129.Rajkowska G., Mïguel-Hïdalgo JJ., Wei J., et al. Morphometry evidence for neuronal and glia pre-frontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 130.Krïshnan K., Doraïswamy P., Lurïe S., et al. Pituitary size in depression. J Clin Endocrinol. 1991;72:256–259. doi: 10.1210/jcem-72-2-256. [DOI] [PubMed] [Google Scholar]
- 131.Nibuya M., Takahashi M., Russell DS., Duman RS. Chronic stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett. 1999;267:81–84. doi: 10.1016/s0304-3940(99)00335-3. [DOI] [PubMed] [Google Scholar]
- 132.Wu H., Friedman WJ., Dreyfus CF. Differential regulation of neurotrophin expression in basal forebrain astrocytes by neuronal signals. J Neurosci Res. 2004;76:76–85. doi: 10.1002/jnr.20060. [DOI] [PubMed] [Google Scholar]
- 133.Klein J. Functions and pathophysiological roles of phospholïpase D in the brain. J Neurochem. 2005;94:1473–1487. doi: 10.1111/j.1471-4159.2005.03315.x. [DOI] [PubMed] [Google Scholar]
- 134.Guillemin GJ., Brew BJ., Noonan CE., et al. Indoleamine 2,3 dïoxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 135.Egashïra N., Iwasaki K., Takashïma A., et al. Altered depression-related behaviour and neurochemical changes in serotonergic neurons in mutant R 406W human tau transgenic mice. Brain Res. 2005;1059:7–12. doi: 10.1016/j.brainres.2005.08.004. [DOI] [PubMed] [Google Scholar]