Abstract
Brain serotonergic circuitries interact with other neurotransmitter systems on a multitude of different molecular levels. In humans, as in other mammalian species, serotonin (5-HT) plays a modulatory role in almost every physiological function. Furthermore, serotonergic dysfunction is thought to be implicated in several psychiatric and neurodegenerative disorders. We describe the neuroanatomy and neurochemistry of brain serotonergic circuitries. The contribution of emergent in vivo imaging methods to the regional localization of binding site receptors and certain aspects of their functional connectivity in correlation to behavior is also discussed. 5-HT cell bodies, mainly localized in the raphe nuclei, send axons to almost every brain region. It is argued that the specificity of the local chemocommunication between 5-HT and other neuronal elements mainly depends on mechanisms regulating the extracellular concentration of 5-HT, the diversity of high-affinity membrane receptors, and their specific transduction modalities.
Keywords: 5-hydroxytryptamine, raphe nucleus, serotonin receptor, neuroanatomy, in vivo imaging, human brain
Abstract
Los circuitos serotoninérgicos cerebrales interactúan con otros sistemas de neurotransmisión en una infinidad de diferentes niveles moleculares. En humanos, como también en otras especies de mamíferos, la serotonina (5HT) tiene un papel modulador en casi todas las funciones fisiológicas. Además se postula que la disfunción serotoninérgica participa en diversos trastornos psiquiátricos y neurodegenerativos. Se describe la neuroanatomía y la neuroquímica de los circuitos serotoninérgicos cerebrales. También se discute la contribución de novedosos métodos de imágenes in vivo para la localización regional de sitios de unión de receptores y ciertos aspectos de su conectividad funcional en relación con la conducta. Los cuerpos de 5-HT, localizados principalmente en los núcleos del rafe, envían axones a casi todas las regiones cerebrales. Se argumenta que la especificidad de la comunicación química local entre 5-HT y otros elementos neuronales depende principalmente de mecanismos que regulan la concentración extracelular de 5-HT, de la diversidad de receptores de membrana de alta afinidad y de sus modalidades de transducción específicas.
Abstract
Les circuits sérotoninergiques centraux sont le théâtre d'une myriade d'interactions moléculaires dévolues à leur communication. Chez l'homme comme chez les autres espèces, la sérotonine (5-HT) joue un rôle modulateur dans la presque totalité des fonctions physiologiques. De plus, un dysfonctionnement des systèmes sérotoninergiques est présumé impliqué dans diverses pathologies psychiatriques et neurodégénératives. Nous décrivons en détail les circuits sérotoninergiques centraux à partir d'études neuroanatomiques postmortem. La contribution des approches modernes in vivo permettant la localisation régionale de récepteurs et certains aspects de leur fonctionnalité corrélée à des comportements sont aussi discutées. Les corps cellulaires à 5-HT principalement localisés dans les noyaux des raphés projettent des axones dans la plupart des régions du cerveau. Ainsi la spécificité de la communication chimique locale établie entre les éléments neuronaux à 5-HT et les autres dépend de mécanismes régulant la concentration extracellulaire en 5-HT, de la diversité des récepteurs membranaires de haute affinité et de leurs modalités de transduction.
Serotonin or 5-hydroxytryptamine (5-HT) is a small indolamine (MW 176.2) widely distributed throughout the animal (from ascidies to human)1-4 and plant5,6 kingdoms. In mammals, a gut-stimulating factor called enteramine, distinct from subtance P, was reported in 1940.7-9 Eight years later, a vasoconstrictor factor named serotonin was isolated from the serum.10 It was subsequently demonstrated that enteramine and serotonin were the same chemical entity, ie, 5-HT. The biological activity of 5-HT in peripheral nerves and brain was described a few years later.11-15 Additionally, developmental studies reveal that 5-HT occurs early during fetal life and plays a role in morphogenesis as well as in neural trafficking.16,17
Among the large variety of chemical messengers acting in nerve cell signaling, 5-HT is the focus of much interest due to its implication in almost every physiological function (eating, reward, thermoregulation, cardiovascular regulation, locomotion, pain, reproduction, sleepwake cycle, memory, cognition, aggressiveness, responses to stressors, emotion, and mood) and in several human pathologies. Thus, dysfunction of the serotonergic systems is thought to be associated with irritable bowel syndrome,18 restless legs syndrome,19 sudden infant death syndrome,20,21 autism,22 headache,23 insomnia,24 anxiety,25 depression,26 anorexia,27,28 schizophrenia,29 Parkinson's disease,30 and Alzheimer's disease.31,32 At the present time, most of the anxiolytic/antidepressant compounds such as tricyclic and tetracyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs),33,34 azapirones,35 setron antiemetics,36 and triptans used to relieve migraine,37 all target the serotonergic systems. Besides a well-known dopaminergic component, atypical neuroleptics (eg, olanzapine, clozapine, quetiapine, aripiprazole) interact with serotonergic receptors (ie, 5-HT1A, 5-HT2A-2C, 5-HT6 and 5-HT7).38-40 Finally, psychotropic drugs including LSD, mescaline, cocaine, and amphetamines powerfully alter 5-HT functions via 5-HT1A, 5-HT2A receptors41,42 and monoaminergic transporters.43-45
5-HT is massively synthesized in the gastrointestinal tract (mainly in enterochromafin cells), whereas only a small percentage is produced within the nervous system.46,47 There is some evidence that 5-HT synthesis, release by calcium-dependent exocytosis, selective reuptake by an energy-dependent membrane transporter, metabolism and reuptake in vesicles operate in all the neuronal elements of the 5-HT neurons (ie, soma, dendrites, axons, and terminals), together participating in 5-HT homeostasis.48,49 The widespread distribution of 5-HT axons and terminals throughout the neuraxis (Figure 1), the frequent nonsynaptic neurotransmission (called diffuse or volume neurotransmission 48,50-52), as well as the abundance of 5-HT receptors (Table I) contribute to explaining the complex relationships between 5-HT and other neurotransmitter and neurohormonal systems.
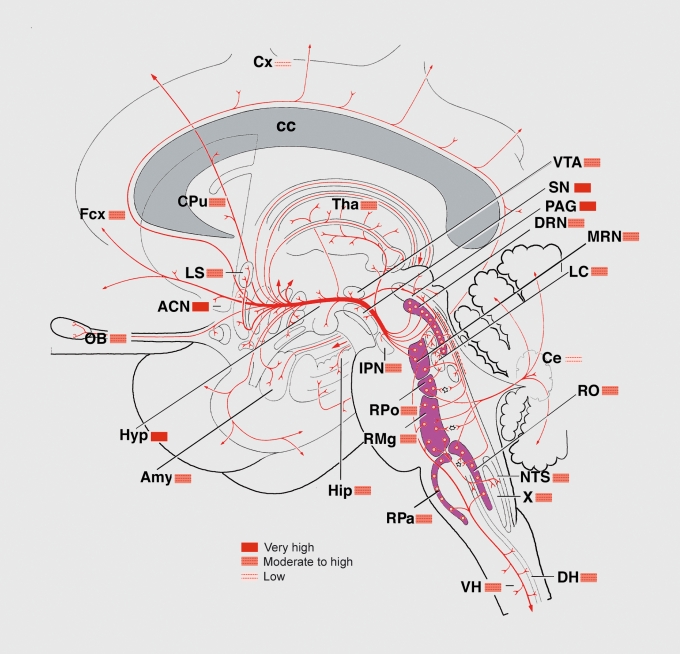
Figure 1. Schematic sagittal view of the human brain showing the distribution of the serotonergic systems. The raphe nuclei containing the majority of the serotonergic cell bodies appear in purple. It is readily seen that these nuclei are exclusively located in the brain stem. The axons issued from them are drawn in red. The trajectories and extensive branching of the axons until the main terminal areas are illustrated. The densities of the serotonergic axonal networks in these terminal areas are given by the colored boxes. X, dorsal motor n of the vagus nerve; ACN, accumbens n; Amy, amygdala; cc, corpus callosum; Ce, cerebellum; CPu, caudate-putamen; Cx, cortex; DH, dorsal horn spinal cord; DRN, dorsa raphe n; Fcx, frontal cortex; Hip, hippocampus; Hyp, hypothalamus; IPN, interpeduncular n; LC, locus coeruleus; LS, lateral septum; MRN, median raphe n; n, nucleus; NTS, n of the solitary tract; OB, olfactory bulb; PAG, periaqueductal gray; RMg, raphe magnus n; RO, raphe obscurus n; Rpa, raphe pallidus; RPo, raphe pontis n; SN, subtantia nigra; Tha, thalamus; VH, ventral horn; VTA, ventral tegmental area Adapted from ref 129: Nieuwenhuis R. Monoamines: Chemoarchitecture of the Brain. Berlin, Germany: Springer Verlag; 1985:33-41. Copyright © Springer Verlag, 1985 .
Table I. Table I. Serotonin (5-HT) receptors in the human brain: distribution, putative functions, and related pathologies. Pre-RNA *splicing and ° editing variants. For review see also refs 98 to100. X, dorsal motor n of the vagus nerve; ACN, accumbens n; Amy, amygdala; cc, corpus callosum; Ce, cerebellum; CPu, caudate-putamen; Cx, cortex; DRN, dorsal raphe n; Fcx, frontal cortex; Hip, hippocampus; Hyp, hypothalamus; LS, lateral septum; MRN, n, nucleus; SN, subtantia nigra; Tha, thalamus; VTA, ventral tegmental area.
| 5-HT receptor | Locus | Aminoacid length | Human brain regions | Putative functions | Related clinical interests | Ref |
| 5-HT1A | 5q11.2-q13 | 422 | Raphe n hyp, hip, amy, CPu, Cx, Fcx | 5-HT activit ermoregulatio eding, stress, pai od, emotion, cognitio arning, memory.. | Anxiety/depression, neurodegenerative disorders, schizophrenia | 25,147,171 |
| 5-HT1B (5-HT1Dß) | 6q13 | 390 | SN /VTA, ACN, CPu, ventral pallidum, Cx | 5-HT activity mood, feeding | Anxiety/depression, migraine | 131,138,172 |
| 5-HT1D | 1p36.3-34.3 | 343 | CPu, , ventral pallidum, Fcx | 5-HT activity, mood, feeding | Anxiety/depression, migraine | 173 |
| 5-HT1E | 6q14-q15 | 365 | CPu, Hyp, Cx | (?) | (?) | See 174 |
| 5-HT1F | 3p13-p14.1 | 366 | Ce, Hip, Cx | Mood, emotion | Migraine | 175 |
| 5-HT2A | 13q14-q21 | 471 | Dorsal vagal complex, | Mood, respiratory control, | Schizophrenia, anxiety/ | 110,160,176 |
| hypoglossal n, inferi vary complex, Tha u, Cx, FCx | feeding, nociception | depression, Tourette's syndromzheimer's didease, anorexia/ bulimia, drug abuse, pain | ||||
| 5-HT2B | 2q36.3-q37.1 | 481 | Ce (?), LS (?), Hyp (?) Cx (?) | Brain development (?), feeding (?) | Drug abuse, anxiety (?) | 177 |
| 5-HT2C | Xq24 | 458° | Choroid plexus, Ce, DRN, SN, Hyp, Amy, Hip, CPu, ACN, Cx | Mood, impulsivity, feeding, locomotor activity | Anxiety/depressio hizophreni ug abuse, obesity | 178 |
| 5-HT3A-E subunits | 11q23. 1-27.1 | 510* (5-HT3A) | Dorsal vagal complex, Hip, Amy, CPu | Vomiting reflex, mood, | Nausea, anxiety/depression | 103,104 |
| 5-HT4 | 5q34-q36 | 402* | Hyp, Hip, ACN, CPu | Feeding, reward, cognition | Anorexia, drug abuse, Alzheimer's disease | 139,171, 179,180 |
| 5-HT5A | 7q34-q36 | 357 | Ce, Hyp, Thal, Hip, Cx | Circadian rhythm, sleep, mood, cognition | Schizophrenia (?) anxiety/depression (?) | 181 |
| 5-HT6 | 1p36-p35 | 440 | Hip, CPu, Cx, olfactory tubercle | Cognition, learning, memory, feeding | Alzheimer's disease, dementia, obesity | 171,182 |
| 5-HT7 | 10q21-q24 | 479* | Raphe n., Hyp, Tha, Hip, Amy, Cx | Mood, sleep, cognition | Anxiety/depression, schizophrenia. | 183 |
The main goal of this review is to discuss the most salient features concerning the neuroanatomy of the serotonergic neurotransmission, ie, the serotonergic circuitries in the human brain. In the first instance, proteins such as enzymes, transporters, and receptors more specifically devoted to the serotonergic functions will be described. Methodological limits of the classical postmortem approaches in the human and new 5-HT in vivo imaging modalities will also be considered. At the present time, more than 100 000 scientific publications concern 5-HT (PubMed). Wherever possible, we have tried to include up-to-date references dealing with the human brain.
The main molecular protagonists in 5-HT neurotransmission
From tryptophan to serotonin
In the brain, neuron subpopulations have a set of enzymes permitting the two-step synthesis of 5-HT from its precursor tryptophan, an essential aminoacid provided by nutrients and actively cotransported with other neutral large amino acids from the blood to the brain.53 The consequences of tryptophan depletion or loading on physiological functions, including memory, cognition, mood, facial expression of emotion, and sleep, have been reported in detail elsewhere.53-56 Contrasting with the peripheral glandular serotonergic systems (eg, the enterochromafin cells or the pineal gland) that uses a first tryptophan hydroxylase form (TPOH1), 5-HT synthesizing neurons in the brain express another tryptophan hydroxylase (TPOH2) recently evidenced from knockout studies in mice.57 The respective sequences of these isoenzymes revealed 30% heterology, offering the perspective of a selective modulation by appropriate drugs in central or peripheral pathologies.57 Some 5-HT-related neuropsychiatric disorders are possibly correlated with genetic variants of TPOH2.57-61 Additionally, recent analyses indicate that TPOH1 polymorphisms could increase susceptibility to schizophrenia62 and suicidal behavior.63 5-hydroxytryptophan formed during the first rate-limiting step by TPOH1 or TPOH2 is then transformed into 5-HT via an aromatic L-amino acid decarboxylase (AADC) also present in catecholaminergic neurons. Rare AADC point mutations reported in humans result in deficiency of catecholamines and serotonin with severe neuropsychiatric symptoms.64
In the nervous system, 5-HT is mainly metabolized by the monoamine oxidase A (MAOA) and a 5-HT half-life of only a few minutes is reported.65 Thus, reciprocal 5-HT exchanges between the central nervous system (CNS) and other tissues appear to be limited, although a brain 5-HT efflux through the blood-brain barrier was observed in rat species.66 Abnormality in 5-HT metabolites, especially low 5-hydroxyindolacetic acid (5-HIAA) levels in the cerebrospinal fluid (CSF) was correlated with suicidality and severity of aggressive behaviour.67,68 Furthermore, an association between CSF 5-HIAA and cholesterolemia was described in certain suicidal patients.69,70 Although largely conjectural, the neurobiological basis of these observations might be found in the evolution history, a propensity to aggressive behavior in man being related to an ancestral adaptative response to a low-cholesterol diet occurring during starvation and famine.71
Serotonin transporter
The main physiological role of a 5-HT transporter is the clearance of released 5-HT from the extracellular space, and thus the control of the duration and magnitude of neurotransmission via 5-HT receptors. Although an active concentrating mechanism of 5-HT by human platelets was already mentioned by Hardisty and Stacey in 1955 ,72 selective 5-HT uptake into nerves was only reported at the end of the 1960s. Later, it was observed that certain neuronal subpopulations in brain selectively concentrate exogenous tritiated monoamines by uptake.73-75 The binding of anti-depressants to neurons, platelets, gastrointestinal, pulmonary, and placental brush-border membranes bearing a serotonin transporter (SERT or 5-HTT) was then demonstrated.76,77 More than 30 years later, a large family of neurotransmitter sodium symporters was identified by molecular cloning.44 Contrary to metabotropic receptors displaying seven transmembrane domains, the predictive topology of monoamine transporters indicated 12 transmembrane domains, a large extracellular loop, and intracellular N and C terminal sequences. The identification of the human SERT sequence as an antidepressant and a cocaine -sensitive transporter78 in 1993 was just preceded by the description of y-aminobutyric acid (GABA) and noradrenaline transporter sequences. Interestingly, in 1991, Hoffman and coworkers had already reported a SERT sequence from a rodent leukemia cell line.79 SERT homologous sequences were also described in invertebrates such as Drosophila, suggesting that this gene is phylogenetically ancient.80 In humans as well as in other mammalian species, SERT mRNA expression in the brain is restricted to 5-HT cell bodies.81,82 The unique SERT gene includes 14 exons encoding both a short and a long variant in humans and is localized in the long arm of chromosome 17.78 Several polymorphisms, especially in the promoter region of SERT, are presumed to be associated with psychiatric illness including depression, anxiety, cognitive impairment, eating disorders, alcohol dependence, and primary insomnia.83-87
A transcription factor, Pet-1, influences TPOH2 and SERT expression levels in the rodent brain. It was demonstrated that Pet-1 -null mice have severe deficiency in 5-HT signaling associated with anxiety-like and aggressive behaviors.88 However, the role of the human ortholog gene FEV (Fifth Edwin Variant) is less well established.89 Furthermore, it was recently reported that the level of SERT expression is under influence of a micro RNA (MiR-16) upregulated by antidepressants such as fluoxetine.90
As described for other monoamine transporters, reuptake of 5-HT by SERT is ATP-dependent. It was suggested that SERT-associated proteins (a variety of phosphatase and phosphokinase proteins, nNOS and several others) could regulate the transporter velocity, its downregulation by intracellular sequestration, and its surface membrane targeting.77-91
Following its reuptake into the neuronal elements by SERT, 5-HT can be degraded by MAO associated with the mitochondrial membranes. Alternatively, 5-HT is packaged into vesicles by a (H+)-dependent carrier called vesicular monoamine transporter 2 (VMAT2) also present in other monoaminergic neurons. The factors leading to the packaging rather than degradation of 5-HT within 5-HT neurons remain to be elucidated. Very intriguing is the recent report of vesicular-filling synergy in serotonergic neurons, a mechanism previously found in certain cholinergic neurons.81 Thus, it was observed that half of the neocortical and hippocampal subsets of 5-HT neuronal elements lacking SERT coexpress VMAT2 and the vesicular glutamate transporter VGLUT3 on the same vesicles. It was further demonstrated that vesicular glutamate uptake via VGLUT3 allows 5-HT vesicular filling by VMAT2, fostering 5-HT release from tonically active terminals involved in volume transmission. Serotonergic fibers and terminals coexpressing VGLUT3 and VMAT2 but lacking reuptake by SERT could represent sites of powerful regulatory mechanisms in 5-HT neurotransmission (for further details see ref 81). VMAT2 is targeted by several psychoactive drugs such amphetamines, tetrabenazine, and reserpine, which finally facilitate 5-HT depletion within neurons by its release in the extracellular space.49 Specific haplotypes in the VMAT2 gene are possibly associated with depression symptoms.92 They are also presumed to be protective in Parkinson's disease93 and alcoholism.94
Serotonin receptors
The first evidence for 5-HT/tryptamine receptors and their desensitization were reported in the guinea-pig ileum during the 1950s. According to their sensitivity to morphine or dibenzyline, 5-HT/tryptamine receptors were called M and D, respectively. It was further suggested that M receptors also act in the nervous system.95 The presence of 5-HT receptors in the brain was deduced from electrophysiological and pharmacological investigations in the cat lateral geniculate nucleus. Thus, it was demonstrated that lysergic acid diethylamide (LSD) directly influences central 5-HT receptors. Based on binding experiments of [3H]5-HT and [3H]spiroperidol, two distinct 5-HT receptor populations (5-HT1 and 5-HT2) were described in rodent and bovine brain membranes.96 On pharmacological criteria, four brain 5-HT 1 receptor subtypes (5-HT1A, 5-HT1B, 5-HT1C, 5-HT1D) and a peripheral 5-HT3 serotonin receptor were then described in rodents.97 From 1987 to the present time, more than 15 5-HT receptors grouped into seven families were identified by various cloning strategies and characterized as distinct entities encoded by distinct genes (Table I). Additional pre-RNA splicing and editing variants were further demonstrated for 5-HT2C, 5HT3A, 5-HT4, and 5-HT7 receptors.98 The same 5-HT receptor diversity was also observed in humans (Table I) and other mammalian species, although interspecies differences in their neuroanatomical distribution or their pharmacological profiles were noted.
With a few exceptions, the 5-HT receptor subtypes are expressed in the nervous system98-100 as well as in the gastrointestinal tract.46,47,101,102 5-HT3 receptors103,104 are ionotropic receptors formed by a pentamer of subunits (mainly 5-HT3A and B), whereas the other 5-HT receptors are metabotropic (G-protein coupled receptors) activating a large variety of signaling pathways.105,106 As expected, the growing number of 5-HT receptor subtypes stimulates the development of selective interactive compounds of potential interest as therapeutic agents and, more recently, radiopharmaceutical tracers for in vivo imaging. It can be noted that the in silico design (ie, computer simulation) of these compounds gains more and more importance (for example see ref 107).
5-HT receptor subtypes more often coexist in the brain areas enriched in 5-HT-neuronal elements (Table I, Figure 1). In the human brain, like in other species, the substantia nigra, the hippocampal formation, the hypothalamus, the amygdala, the striatum, and the frontal cortex display a large set of 5-HT receptors. Their relative densities show great variation among the brain areas, some of them being highly expressed in a restricted number of regions (eg, 5-HT3, 5-HT4, 5-HT6). Our knowledge of the anatomical distribution of 5-HT receptors in the human brain is not exhaustive, since selective ligands or specific antibodies for certain 5-HT receptor subtypes are not yet available (eg, 5-HT1E, 5HT2B, 5-HT5A receptors). Consequently, their distribution is only based on their respective mRNA expression obtained by in situ hybridization histochemistry, and thus remains less well characterized.
From pharmacological characterization in human and basic studies in animal models there is evidence that 5-HT receptor density at the surface of the neuronal elements and their activity vary. A sustained stimulation of 5-HT receptors by agonist or endogenous 5-HT results in attenuated receptor responsiveness (or desensitization), intracellular sequestration (or internalization) and receptor recycling back to the membrane (eg, see refs 108, 109). Such mechanisms involve the activation of protein kinase C, phospholipase D and binding to arrestin proteins, uncoupling the transduction by G-protein subunits.105,106 When stimulated by released 5-HT or 5-HT agonists, somatodendritic 5-HT1A autoreceptors in the raphe nuclei and 5-HT1B/1D autoreceptors in 5-HT terminal areas represent a powerful feedback mechanism, decreasing both the firing of the 5-HT neurons and the release of the neurotransmitter. Besides other neuroplastic changes, longterm desensitization and sequestration of these 5-HT receptor subtypes could be implicated in the delayed response of anxiolytic/antidepressants (SSRIs, buspirone, etc). Perhaps of special interest in psychosis, heterologous desensitization of 5-HT1 A receptors by 5-HT2A receptor activation and close relationships between 5-HT, SERT, and 5-HT2A receptor densities were recently demonstrated in the living human brain.110 Desensitization is not restricted to metabotropic receptors. Indeed desensitization of 5-HT3 receptor channels following sustained stimulation may play a critical physiological role in the regulation of neuronal excitability via this receptor.111
Intriguingly, homodimerization between 5-HT receptors (eg, 5-HT2A, 5-HT2C, 5-HT4 receptors) or even heterodimerization, an aggregate of two unrelated receptors, such as a 5-HT2A/ metabotropic glutamate receptor 2 dimerized complexes integrating both 5-HT and glutamate signaling, were reported in the human cortex.112 Furthermore, this complex could increase the affinity of 5-HT2A receptors for hallucinogenic compounds such as LSD.113 It was also recently reported that the internalization of CRF1 receptors by a CRF agonist enhances 5-HT2A signaling and anxiety-related behavior by recycling this receptor to the plasma membrane from an intracellular pool.114,115 Finally, a variety of proteins including (3-arrestins, serine/threonine protein kinases, protein phosphatase and tensin homolog, calpactin, and PDZ proteins interact with 5-HT receptor subtypes, modifying their functional activity105,116 They represent putative new targets for treatment of mood disorders and addiction.
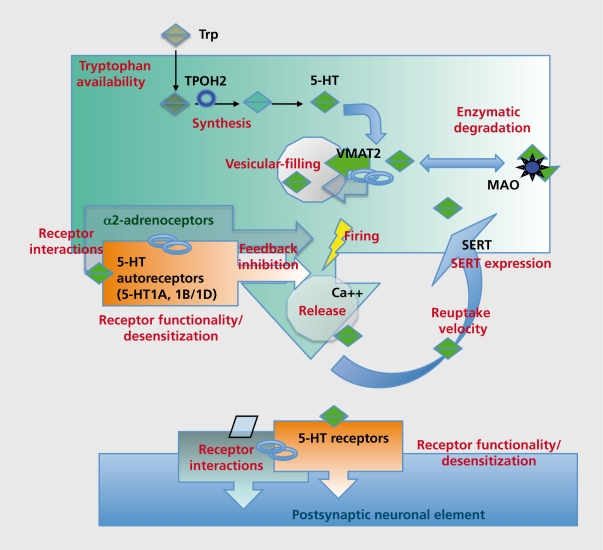
Thus, the status and function of 5-HT receptors in the brain depend on a multiplicity of factors including crosstalk with other homologous and heterologous receptors.106 As illustrated in (Figure 2) 5-HT availability in the extracellular space and target receptor functions are regulated at multiple levels, some of them being closely linked (eg, 5-HT1A, 5-HT1B/1D feedback mechanisms).
Figure 2. The serotonergic neurotransmission depends on serotonin (5-HT) levels present in the extracellular space and on membrane receptors triggering functional changes in neighbouring neuronal elements. 5-HT synthesis, release and reuptake are regulated by several mechanisms including feedback inhibition by 5-HT1A, 5-HT1B/1D autoreceptors and a-2 adrenoceptors. Other mechanisms of regulation are receptor dimerization and desensitization affecting their trafficking and functionality. See text for further details.
Anatomical organization of 5-HT circuitries in the brain
Morphological approaches in the brain
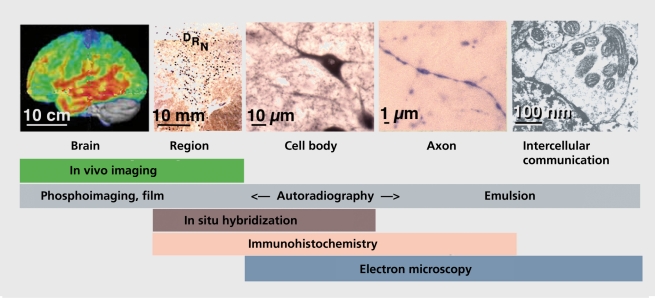
The respective scales of morphological approaches in the brain are called in Figure 3. Thus, imaging of the human living brain provides nowadays an incredible amount of information on functionally linked regions and, according to the availability of selective radiotracers, on millimetric clusters of binding sites. Morphological approaches including immunohistochemistry, in situ hybridization histochemistry and autoradiography allow to visualize a nucleus like the dorsal raphe, as well as a single labeled neuronal element of approximately one micrometer in diameter (eg, an axon varicosity) in brain tissue sections (Figure 3). Electron microscopy studies in the human brain and, more often, in other mammalian species give ultrastructural details (eg, junctions between neuronal elements or 5-HT1A receptor internalization).117
Figure 3. Photographs illustrating the different scales provided by the different anatomical methods used to investigate the brain. In vivo imaging allows regional analyses (from the whole brain to groups of neurons), whereas electron microscopy provides images of neuronal cell bodies and is particularly useful to visualize axonal varicosities and their contacts with neighboring elements. In between are autoradiography in situ hybridization, and immunohistochemistry. DRN, dorsal raphe nucleus.
Cellular mapping of 5-HT-producing neurons in the CNS
Due to the postmortem instability of 5-HT118 and other possible methodological bias,119 quantitative biochemical estimation of 5-HT in the human brain subdivisions should be interpreted with caution, as illustrated by the numerous discrepant data reported since the 1950s. For the same reason, morphological approaches by formaldehyde-induced fluorescence or immunohistochemistry using antibodies against 5-HT are limited to biopsies and fetal brain tissues. Most of the anatomical studies in human are based on regional autoradiography of SERT binding sites to selective radioligands and immunohistochemical studies using antibodies against TPOH, which represent more stable postmortem markers. Therefore, from these studies and those performed in much detail in other species including rodents,120 cat,121 and nonhuman primates,122 it appears that the anatomy of the serotonergic system has remained somewhat similar between different species of mammals.
The 5-HT systems belong to the neuronal systems composed of a restricted number of neurons emitting extensively branched, non- or poorly myelinated axons that innervate almost all brain nuclei. As first described in human fetuses123,124 and later in adults by several authors,125-129 the distribution of the 5-HT cell bodies (approximately 350 000 cells) in the human brain is restricted to the brain stem. As illustrated in Figure 1, a large majority of them is concentrated along the midline in the raphe nuclei, extending from the caudalmost level of the medulla oblongata to mid-level of mesencephalon, but a substantial number is located in the reticular formation lateral to these nuclei. The 5-HT neurons form a continuum of cells with loosely defined boundaries along the raphe nuclei. On the basis of studies of cell body localization and their respective projections, the 5-HT neurons can be separated into two groups: a rostral group located in the mesencephalic and rostral pons, sending axons to the forebrain, and a caudal group lying in the rostral pons and medulla oblongata, sending axons in the brain stem and spinal cord (refs in ref 128) In humans, the rostral group contains approximately 85% of the 5-HT neurons. It is composed of neurons located in four nuclei and one area, namely the interpeduncular, the caudal linear, the dorsal raphe (DRN with 165 000 neurons) and the median raphe (MRN with 64 000 neurons) nuclei. The additional area corresponds to the caudal mesencephalic and rostral pontine reticular formation. 5-HT neurons spread in this area were already observed in the rat and cat species and their large number estimated in human (60 000 neurons).
The caudal group accounts for 15% of all the 5-HT neurons. It is composed of 5-HT neurons located in three raphe nuclei, namely the raphe magnus (30 000 neurons), the raphe obscurus, and the raphe pallidus (1000 neurons), and in the ventral medullary reticular formation lying lateral to the raphe magnus and the pyramids. As noted earlier, the rostral and caudal groups have separate afferent projections, with, however, some overlapping in the brain stem and as far down as the spinal cord. The trajectories of the efferent pathways have been studied in laboratory animals, often combining retrograde tracing with immunohistochemistry. Thus, a rostral and a ventral pathway emerge from the rostral group, rapidly join ventrally and split again into a lateral projection running in the internal capsule to innervate the lateral cortex and a longitudinal rostral projection running in the medial forebrain bundle to innervate the hypothalamus, basal forebrain, septum, basal ganglia, and amygdala. This rostral projection extends into the cingulum and innervates the medial cortex and the hippocampus.
The density of innervation in terminal areas reported in certain human brain areas has been extensively studied in cat and rodents. This density greatly varies from one region to the other and also within a region (Figure 1). In the cerebral cortex, the superficial layer receives more axons than the other layers. A dense innervation is observed in the ventromedial part of the caudate-putamen and in the globus pallidus. Ventral to them, the subtantia innominata is also richly supplied in 5-HT terminals. In the amygdala, the basal nucleus stands out for its very high number of 5-HT axons. In humans, like in animals, the 5-HT axons innervating the cortex and the hippocampus display two different morphologies.130 One category of axons bears spaced small and elongated varicosities while the other category displays closely spaced, large, and round varicosities. It can be noted that the two populations of axons show several interesting properties. First, they are respectively issued from two different raphe nuclei, the DRN and the MRN. Second, the small varicose axons correspond to the numerous 5-HT axons not engaged in true synaptic contacts. For example, it is remarkable that only 5% of the varicosities display synapses in the rat frontoparietal cortex.48 Thirdly, and of special clinical interest, the small varicose axons are more susceptible to degeneration caused by amphetamine derivatives, like ecstasy.131 The caudal group of 5HT neurons sends axons both laterally in the reticular formation and downwards in the spinal cord. In the reticular formation, the 5-HT axons are particularly abundant in the cranial motor nuclei (trigeminal, facial and hypoglossal). In the spinal cord, the 5-HT axons terminate in all subdivisions and along the whole length of the cord. In the dorsal horn, the superficial layers are densely innervated. In the intermediate gray, the preganglionic sympathetic neurons of the intermediolateral column are densely surrounded by 5-HT axons. In the ventral horn, the 5-HT axons are in close apposition to the motor neurons, especially in primates.132
In vivo imaging of the brain serotonergic systems
Structural and functional tomography through the living brain is currently possible. Powerful tools, such as positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), and pharmacological MRI (phMRI),133-135 add new information on the functional anatomy of the serotonergic systems in the human brain. PET and SPECT neuroimaging respectively use positron-emitting nuclides (18F, 11C) and gamma-emitters (123I, 125I) coupled to a small heterocyclic compound selective for one 5-HT receptor subpopulation, SERT or MAO A.87,136,137 Since the radiotracer is injected at trace level, 5-HT receptors or SERT can be localized in vivo and their relative concentration/affinity estimated from binding potential (BP). A submillimeter spatial resolution is commonly reported in PET and SPECT studies. However, at the present time very few radiotracers selective for SERT, 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT4 receptors are available.87,134,136-140 The design of new radiopharmaceuticals for in vivo imaging is constrained by several criteria including brain penetrability, target selectivity, and the absence of troublesome radiometabolites.141 Additionally, when using radiolabeled glucose analogs, PET and SPECT modalities provide information on blood flow and in some circumstances may reflect a local activity of nervous cells following a specific pharmaceutical treatment (eg, anxiolytics, antidepressants). Offering a better spatial and temporal resolution, phMRI represents another imaging method based on the hemodynamic response to changes in neuronal activity induced by pharmacological manipulations. This emergent imaging modality providing an indirect measure of aggregated neuronal function could have an important impact on future 5-HT research in the living human brain.133,135
Despite the limited number of available radiotracers, in vivo imaging of 5-HT function gains more and more interest in basic research as well as in clinical medicine. For example, recent publications suggest a lateralization of 5-HT1 A binding in language areas (auditory cortices) and sex differences in cortical and subcortical brain areas of healthy subjects.142 A selective interrelation between 5-HT1A distribution, sex hormones, and aggression score in humans was also demonstrated by in vivo imaging and biochemical analyses.143 More intriguingly, PET imaging studies clearly indicate that 5-HT2A receptor binding in the cortex is positively correlated to the body mass index144 and the response in painful heat stimulation.145 Furthermore, it was reported that an inverse relationship between 5-HT2A receptor and SERT BPs in the neocortex might be the result of interindividual differences in baseline 5-HT levels.110 Mainly based on SERT binding, PET studies support a loss of serotonergic pathway integrity in ecstasy users146 and patients suffering from schizophrenia, Alzheimer's and Parkinson's diseases, whereas they were more inconclusive for assessing human depression,.137,147 Further, 5-HT dysfunction due to certain genetic variations in SERT and 5-HT receptor sequences is now detectable by functional neuroimaging.87,150-150
Although not quite completely understood, these recent data from living human brain imaging support and often greatly extend, previous data obtained by conventional postmortem investigations.
Serotonergic circuitries in function
Serotonergic circuitries chiefly include 5-HT-producing neurons, 5-HT-autoreceptors (ie, somatodendritic 5HT1 A receptors, 5-HT1B/1D receptors in terminal endings) and other neurotransmitter or hormone receptors including alpha-adrenoceptors, CRF receptors, tachykinin receptors, estrogen receptor beta and more recently demonstrated, oxytocin receptors151 involved in neuronal firing and 5-HT release. Functionally connected neuronal elements bearing 5-HT-heteroreceptors (often called postsynaptic or perisynaptic receptors, see below) are obviously another major component of the serotonergic neurotransmission.100,152,153 Additionally, classical neurotransmitters (eg, GABA, glutamate, dopamine, noradrenaline), peptidergic neuromodulators (eg, substance P), and endocannabinoid coexpression within 5-HT neurons also contribute to the serotonergic function.154,155
Considering that in several brain areas, including the neocortex and the hippocampus, 5-HT wired neurotransmission (WT) via true synapses coexists with volume transmission (VT), the terms pre- and postsynaptic should be used with caution. In fact, distances between release sites and receptors are not of the same magnitude, generally a few nm for WT vs up to 10 µm for VT. Thus, some authors consider that neuropsychoactive drugs act rather as volume transmission signals.156
Due to ethical and methodological limitations, our knowledge on neurotransmitter circuitries and their interconnections in human CNS largely benefits from that described with much detail in nonhuman primates and other species including cat and rodents. In laboratory animal species, the anatomical distribution of brain 5-HT neurons was often completed by other approaches such as transneuronal retrograde transport, selective lesions, microdialysis, electrophysiology associated with pharmacological manipulations, and more recently developed wireless fast-scan cyclic voltametry, a promising tool for the in vivo monitoring of 5-HT in the brain.157 Therefore, the circuitries of serotonergic neurons in the human brain are mainly based on those known in other mammals. In spite of obvious species differences concerning the relative size and functional development of certain brain structures (eg, certain neocortical subdivisions, the olfactory system), behavioral effects of neurological lesions or other disease processes and neuroanatomopathological studies in human suggest that on the whole, serotonergic circuitries serve comparable basic functions among mammals. However, contemporary neuroimaging technologies mentioned above (especially functional and pharmacological MRI, and PET) combined with behavioral approaches, offer a variety of new opportunities for the investigation of the limbic system in the living human brain.134,149,158,159 Thus, recent articles report the exploration of the corticolimbic circuitries in relation to emotion and cognition.158,160,161 Multimodal in vivo imaging studies add new information on the medial prefrontal cortex and amygdala coupling,160 providing an advanced knowledge on the brain mechanism of certain pathophysiological effects of social anxiety disorder.134
As described above, 5-HT neurons send axons and terminals throughout the entire brain and therefore can potentially interact with almost all the other neuronal systems via the diversity of 5-HT heteroceptors (ie, receptors expressed by neurons that do not synthesize 5HT).100 Recent investigations in mice indicate that other mechanisms could also contribute to the 5-HT signaling. Thus, it was demonstrated that local infusion of fluoxetine (a SSRI) in the dorsal raphe nucleus stimulates the secretion of the protein S100-beta by 5-HT neurons projecting to the locus cereuleus. This protein downregulates the microRNA miR-16 in noradrenergic neurons which in turn switch on serotonergic functions.90
Reciprocally, classical neurotransmitters, especially GABAergic, catecholaminergic, glutamatergic, cholinergic, and histaminergic systems, influence the serotonergic neurotransmission at different sites, including the raphe nuclei. It is well known that the raphe nuclei contain collections of non-5-HT neuronal elements (eg, GABAergic, glutamatergic, cholinergic, histaminergic, dopaminergic, noradrenergic) interacting with 5-HT cell bodies via their respective receptor subsets.162,163 Moreover, the richness in heteroreceptors (eg, alpha2-adrenoceptors, glutamatergic, histaminergic receptors) expressed by 5-HT terminals and other local mechanisms (eg, vesicular-filling synergy) mentioned above illustrate the extent of the reciprocal chemocommunication between serotonergic circuitries and other neurotransmitter networks.
Other interactions of clinical importance concern the interaction between serotonergic neurotransmission and neuropeptidergic systems. It is well known that 5-HT influences the activity of the hypothalamo-pituitary-adrenal axis at multiple levels, playing a role in stress-related disorders. Thus, 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT2C receptor agonists enhance CRH and ACTH secretion and, consecutively, cortisol and other hormone levels in the plasma.164,165 In turn, corticosteroids attenuate the activity of 5-HT1 A receptors in the dorsal raphe nucleus, the hippocampal formation and the frontal cortex. Many other interactions between the serotonergic and the peptidergic systems (eg, ACTH, cholecystokinin, CART peptide, neuropeptide Y, ghrelin) are implicated in the sleep-wake rhythm and feeding. Other factors known to locally influence 5-HT neurotransmission are neurosteroids (eg, progesterone in the hypothalamus),166 lipids,167 and neurotrophic factors (eg, BDNF in the hippocampus).168
Although not exhaustive, most all of the reciprocal interactions exemplified above involve specialized receptors.
Concluding remarks
It is conceivable that the list of molecular factors that act in 5-HT circuitries is still incomplete. The discovery of TPOH2 is less than 7 years old. Intriguingly, a very recent study in double (TPOH1/TPOH2) knockout mice mentioned a residual 5-HT synthesis, suggesting additional 5-HT synthetic pathway(s).169 Further, it can reasonably be assumed that 5-HT receptor subtypes resulting from postranslational editing or alternative splicing mRNA are not restricted to 5-HT2C, 5-HT3, 5-HT4, and 5-HT7 receptor families. There is also a growing list of proteins playing a role in the regulation of SERT and 5HT receptor activity. Beyond the diversity of 5-HT receptor subtypes, their crosstalk modalities, and their local ability for adaptation, volume transmission demonstrated in several brain regions adds to the complexity of the serotonergic circuitries. Such complexity may explain why small subpopulations of cell bodies sending axons throughout the entire brain may produce such a large spectrum of effects in brain functions. Molecular and cellular studies in laboratory animal models (mutant mice, Caenorhabditis elegans, cell lines) and postmortem human brain have enabled us to explore the serotonergic system and will certainly continue to do so.
Undoubtedly, improvement of the specificity and spatiotemporal resolution of in vivo imaging modalities coupled or not to pharmacological manipulations will also significantly contribute to a better knowledge of 5-HT circuitries, specifically in the living human brain. As already mentioned, human brain structures associated with emotional processing, attention, and some other cognitive functions, are currently being investigated by MRI. TEP modalities allow the visualization of receptors including 5-HT receptors. A next step in functional neuroimaging will be hybrid-scanner systems that combine both technologies.170
Finally, our reviewing on brain serotonergic circuitries has not taken into account the next level of complexity, ie, the fact that the role of other neurotransmitters is not limited to the modulation of 5-HT neuron activity.
Appendix - glossary
Autoreceptors/heteroreceptors
Autoreceptors are membrane receptors expressed by neurons that synthesize the neurotransmitter binding to these receptors, eg, 5-HT1A or 5-HT1B localized on 5-HT neuronal elements. In contrast, heteroreceptors are membrane receptors born by neurons that do not produce the corresponding neurotransmitter, eg, alpha2adrenoceptors on 5-HT neuronal elements.
Heterologous desensitization
A sustained stimulation of a receptor by one agonist results in a homologous desensitization of this receptor (eg, 5-HT1A receptor desensitization by buspirone). Heterologous desensitization occurs when the binding of one agonist to a receptor subtype induces the attenuation of another receptor signaling (eg, desensitization of hypothalamic 5-HT1A receptors following 5-HT2A activation, desensitization of 5-HT2A receptors by activation of 5-HT1A receptors in the same region).
Homodimerization/heterodimerization
Most membrane G protein-coupled receptors exist as dimers or oligomers. A complex formed by two identical receptors (eg, 5-HT2A/5-HT2A; 5-HT2C/5-HT2C receptors) is called a homodimer, whereas a complex formed by unrelated receptors is heterodimer (eg, 5-HT2A/ Glutamate receptor 2; 5-HT2A/D2 receptors). Dimerization occurs during transport of newly formed receptors to the cell surface. The homo- or heterodimeric complexes influence the signaling and internalization of receptors.
MicroRNAs
MicroRNA are small noncoding RNAs mediating posttranscriptional gene regulation (mostly translational repression). Thus, it was recently demonstrated that fluoxetine infusion in the dorsal raphe nucleus increases the level of a microRNA called miR-16 and consequently downregulates the mRNA and protein expression of the membrane serotonin transporter.
Somatodendritic receptors
Somatodendritic receptors are localized on the membrane of the cell bodies (soma) and dendrites of neurons, eg, the somatodendritic 5-HT1 A receptors in the dorsal raphe nucleus.
Symporters
A family of membrane molecules coupling the transmembrane movement of a transmitter (monoamine or amino acid) to the transport of ions (mainly Na+, K+ and Cl-). Neurotransmitter transporters (also called neuronal or membrane transporters) play a major role in the regulation of neurotransmission by energy-dependent reuptake of the neurotransmitters from the extracellular space. The neurotransmitter is then recycled by a vesicular transporter (eg, monoamine vesicular transporters) or degraded.
Vesicular-filling synergy
Vesicular-filling synergy (or vesicular synergy) first reported in cholinergic neurons was also detected in 5HT circuitries, especially in limbic areas (hippocampus, prefrontal cortex). The coexpression of a vesicular glutamate transporter (VGLUT3) and a vesicular monoamine transporter (VMAT2) on the same vesicles of 5-HT terminal subpopulations represents a local synergic mechanism between glutamate and 5-HT neurotransmitters. It was demonstrated that glutamate reuptake stimulates vesicular 5-HT accumulation by VMAT2. Thus, 5-HT transmission is locally tuned by glutamate.
Wiring/volume neurotransmission
In wiring neurotransmission the communication between neurons operates via specialized junctional complexes including synapses (intercellular space in the synaptic cleft around 20 nm). The interneuronal communication without junctional complexes is called diffuse (or volume) neurotransmission and was identified in serotonergic, catecholaminergic, cholinergic, and several other transmitter systems. The neurotransmitter released in the extracellular space reaches target receptors localized up to several µm from the source (axon varicosities or terminals). 5-HT volume neurotransmission is frequently observed in the neocortex, the hippocampus, and several other brain areas. For more details on the functional consequences see the references indicated in the text.
Selected abbreviations and acronyms
- 5-HIAA
5 -hydroxyindolacetic acid
- 5-HT
5-hydroxytryptamine
- SERT
serotonin membrane transporter
- TPOH
tryptophan hydroxylase
- VGLUT
vesicular glutamate transporter
- VMAT
vesicular monoamine transporter
See also the Appendix for an explanation of some of the terms used in the text
Contributor Information
Yves Charnay, Département de Psychiatrie, Hôpitaux Universitaires de Genève, Geneva, Switzerland.
Lucienne Leger, Centre National de la Recherche Scientifique, UMR 5167, Faculté de Médecine Laennec, Université C. Bernard, Lyon, France.
REFERENCES
- 1.Barbas D., DesGroseillers L., Castellucci VF., Carew TJ., Marinesco S. Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes. Learn Mem. 2003;10:373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chase DL., Koelle MR. Biogenic amine neurotransmitters in C. elegans. WormBook. 2007:1–15. doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erspamer V. Presenza di enteramina o di una sostanza enteraminosimile negli estratti gastrointestinali e splenici dei pesci e negli estratti gastroenterici delle ascidie. Experientia. 1946;11:369–371. doi: 10.1007/BF02163944. [DOI] [PubMed] [Google Scholar]
- 4.Mathias AP., Ross DM., Schachter M. Identification and distribution of 5-hydroxytryptamine in a sea anemone. Nature. 1957;180:658–659. doi: 10.1038/180658a0. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara A., Hashimoto Y., Tanaka C., et al. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008;54:481–495. doi: 10.1111/j.1365-313X.2008.03441.x. [DOI] [PubMed] [Google Scholar]
- 6.Murch SJ., Alan AR., Cao J., Saxena PK. Melatonin and serotonin in flowers and fruits of Datura metel L. J Pineal Res. 2009;47:277–283. doi: 10.1111/j.1600-079X.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 7.Dalgliesh CE., Toh CC., Work TS. Fractionation of the smooth muscle stimulants present in extracts of gastro-intestinal tract Identification of 5-hydroxytryptamine and its distinction from substance P. J Physiol. 1953;120:298–310. doi: 10.1113/jphysiol.1953.sp004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erspamer V. Pharmakologische studien uber enteramin: Uber die, wirkung von acetonextrkten der kaninchenmagenschleimhaut auf den blutdruck und auf isoliert uberlebende organe. Arch Exp Path Pharmakol. 1940;196:343–407. [Google Scholar]
- 9.Euler Von US., Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapport MM., Green AA., Page IH. Crystalline serotonin. Science. 1948;108:329–230. doi: 10.1126/science.108.2804.329. [DOI] [PubMed] [Google Scholar]
- 11.Brodie BB., Pletscher A., Shore PA. Evidence that serotonin has a role in brain function. Science. 1955;122:968. doi: 10.1126/science.122.3177.968. [DOI] [PubMed] [Google Scholar]
- 12.Amin AH., Crawford TB., Gaddum JH. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J Physiol. 1954;126:596–618. doi: 10.1113/jphysiol.1954.sp005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twarog BM., Page IH. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953;175:157–161. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- 14.Schneider JA., Yonkman FF. Action of serotonin (5-hydroxytryptamine) on vagal afferent impulses in the cat. Am J Physiol. 1953;174:127–134. doi: 10.1152/ajplegacy.1953.174.1.127. [DOI] [PubMed] [Google Scholar]
- 15.Douglas WW., Toh CC. The respiratory stimulant action of 5-hydroxytryptamine (serotonin) in the dog. J Physiol. 1953;120:311–318. doi: 10.1113/jphysiol.1953.sp004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herlenius E., Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev. 2001;65:21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 17.Sundstrom E., Kolare S., Souverbie F., et al. Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res Dev Brain Res. 1993;75:1–12. doi: 10.1016/0165-3806(93)90059-j. [DOI] [PubMed] [Google Scholar]
- 18.Garvin B., Wiley JW. The role of serotonin in irritable bowel syndrome: implications for management. Curr Gastroenterol Rep. 2008;10:363–368. doi: 10.1007/s11894-008-0070-3. [DOI] [PubMed] [Google Scholar]
- 19.Jhoo JH., Yoon IY., Kim YK., et al. Availability of brain serotonin transporters in patients with restless legs syndrome. Neurology. 2010;74:513–518. doi: 10.1212/WNL.0b013e3181cef824. [DOI] [PubMed] [Google Scholar]
- 20.Kinney HC., Richerson GB., Dymecki SM., Darnall RA., Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan JR., Paterson DS., Hoffman JM., et al. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wassink TH., Hazlett HC., Epping EA., et al. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry. 2007;64:709–717. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- 23.Goadsby PJ. Serotonin receptor ligands: treatments of acute migraine and cluster headache. Handb Exp Pharmacol. 2007:129–143. doi: 10.1007/978-3-540-33823-9_5. [DOI] [PubMed] [Google Scholar]
- 24.Jindal. RD. Insomnia in patients with depression: some pathophysiological and treatment considerations. CNS Drugs. 2009;23:309–329. doi: 10.2165/00023210-200923040-00004. [DOI] [PubMed] [Google Scholar]
- 25.Akimova E., Lanzenberger R., Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Nemeroff CB., Owens MJ. The role of serotonin in the pathophysiology of depression: as important as ever. Clin Chem. 2009;55:1578–1579. doi: 10.1373/clinchem.2009.123752. [DOI] [PubMed] [Google Scholar]
- 27.Jean A., Conductier G., Manrique C., et al. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:16335–16340. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye WH., Frank GK., Bailer UF., et al. Serotonin alterations in anorexia and bulimia nervosa: new insights from imaging studies. Physiol Behav. 2005;85:73–81. doi: 10.1016/j.physbeh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen H., Erritzoe D., Andersen R., et al. Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with firstepisode schizophrenia. Arch Gen Psychiatry. 2010;67:9–16. doi: 10.1001/archgenpsychiatry.2009.176. [DOI] [PubMed] [Google Scholar]
- 30.Azmitia EC., Nixon R. Dystrophic serotonergic axons in neurodegenerative diseases. Brain Res. 2008;1217:185–194. doi: 10.1016/j.brainres.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newhouse P., Tatro A., Naylor M., Quealey K., Delgado P. Alzheimer disease, serotonin systems, and tryptophan depletion. Am J Geriatr Psychiatry. 2002;10:483–484. [PubMed] [Google Scholar]
- 32.Ouchi Y., Yoshikawa E., Futatsubashi M., Yagi S., Ueki T., Nakamura K. Altered brain serotonin transporter and associated glucose metabolism in Alzheimer disease. J Nucl Med. 2009;50:1260–1266. doi: 10.2967/jnumed.109.063008. [DOI] [PubMed] [Google Scholar]
- 33.Hamon M., Bourgoin S. Pharmacological profile of antidepressants: a likely basis for their efficacity and side effects. Eur Neuropsychopharmacol. 2006;16:S625–S632. [Google Scholar]
- 34.Racagni G., Popoli M. The pharmacological properties of antidepressants. Int Clin Psychopharmacol. 2010;25:117–131. doi: 10.1097/YIC.0b013e3283311acd. [DOI] [PubMed] [Google Scholar]
- 35.Chessick CA., Allen MH., Thase M., et al. Azapirones for generalized anxiety disorder. Cochrane Database Syst Rev. 2006;3:CD006115. doi: 10.1002/14651858.CD006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito M., Aogi K., Sekine I., et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115–124. doi: 10.1016/S1470-2045(08)70313-9. [DOI] [PubMed] [Google Scholar]
- 37.Law S., Derry S., Moore RA. Triptans for acute cluster headache. Cochrane Database Syst Rev. 2010;4:CD008042. doi: 10.1002/14651858.CD008042.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuroki T., Nagao N., Nakahara T. Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin-dopamine hypothesis. Prog Brain Res. 2008;172:199–212. doi: 10.1016/S0079-6123(08)00910-2. [DOI] [PubMed] [Google Scholar]
- 39.Meltzer HY., Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. 2008;172:177–197. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- 40.Theisen FM., Haberhausen M., Firnges MA., et al. No evidence for binding of clozapine, olanzapine and/or haloperidol to selected receptors involved in body weight regulation. Pharmacogenomics J. 2007;7:275–281. doi: 10.1038/sj.tpj.6500418. [DOI] [PubMed] [Google Scholar]
- 41.Aghajanian GK., Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21 (2 suppl):16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Maeso J., Weisstaub NV., Zhou M., et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. . Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Kish SJ., Fitzmaurice PS., Boileau I., et al. Brain serotonin transporter in human methamphetamine users. Psychopharmacology (Berl). 2009;202:649–661. doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- 44.Sitte HH., Freissmuth M. The reverse operation of Na(+)/Cl(-)-coupled neurotransmitter transporters--why amphetamines take two to tango. J Neurochem. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sucic S., Dallinger S., Zdrazil B., et al. The N terminus of monoamine transporters is a lever required for the action of amphetamines. J Biol Chem. 2010;285:10924–10938. doi: 10.1074/jbc.M109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lesurtel M., Soll C., Graf R., Clavien PA. Role of serotonin in the hepatogastroIntestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 2008;65:940–952. doi: 10.1007/s00018-007-7377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertrand PP., Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153:47–57. doi: 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Descarries L., Beaudet A., Watkins KC. Serotonin nerve terminals in adult rat neocortex. Brain Res. 1975;100:563–588. doi: 10.1016/0006-8993(75)90158-4. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman BJ., Hansson SR., Mezey E., Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- 50.Agnati LF., Guidolin D., Guescini M., Genedani S., Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Descarries L., Mechan A. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurones of the central nervous system. Prog Brain Res. 2000;125:27–47. doi: 10.1016/S0079-6123(00)25005-X. [DOI] [PubMed] [Google Scholar]
- 52.Umbriaco D., Garcia S., Beaulieu C., Descarries L. Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus. 1995;5:605–620. doi: 10.1002/hipo.450050611. [DOI] [PubMed] [Google Scholar]
- 53.Silber BY., Schmitt JA. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci Biobehav Rev. 2010;34:387–407. doi: 10.1016/j.neubiorev.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Daly E., Deeley Q., Hallahan B., et al. Effects of acute tryptophan depletion on neural processing of facial expressions of emotion in humans. Psychopharmacology (Berl). 2010;210:499–510. doi: 10.1007/s00213-010-1850-7. [DOI] [PubMed] [Google Scholar]
- 55.Mendelsohn D., Riedel WJ., Sambeth A. Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev. 2009;33:926–952. doi: 10.1016/j.neubiorev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Moreno FA., Parkinson D., Palmer C., et al. CSF neurochemicals during tryptophan depletion in individuals with remitted depression and healthy controls. Eur Neuropsychopharmacol. 2010;20:18–24. doi: 10.1016/j.euroneuro.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthes S., Mosienko V., Bashammakh S., Alenina N., Bader M. Tryptophan hydroxylase as novel target for the treatment of depressive disorders. Pharmacology. 2010;85:95–109. doi: 10.1159/000279322. [DOI] [PubMed] [Google Scholar]
- 58.Choi KY., Yoon HK., Kim YK. Association between serotonin-related polymorphisms in 5HT2A, TPH1, TPH2 genes and bipolar disorder in Korean population. Psychiatry Investig. 2010;7:60–67. doi: 10.4306/pi.2010.7.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magnay JL., Ismail KM., Chapman G., Cioni L., Jones PW., O'Brien S. Serotonin transporter, tryptophan hydroxylase, and monoamine oxidase A gene polymorphisms in premenstrual dysphoric disorder. Am J Obstet Gynecol. 2006;195:1254–1259. doi: 10.1016/j.ajog.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 60.Mergen H., Demirel B., Akar T., Senol E. Lack of association between the serotonin transporter and tryptophan hydroxylase gene polymorphisms and completed suicide. Psychiatr Genet. 2006;16:53. doi: 10.1097/01.ypg.0000199442.75505.f7. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X., Beaulieu JM., Gainetdinov RR., Caron MG. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell Mol Life Sci. 2006;63:6–11. doi: 10.1007/s00018-005-5417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saetre P., Lundmark P., Wang A., et al. The tryptophan hydroxylase 1 (TPH1) gene, schizophrenia susceptibility, and suicidal behavior: a multicentre case-control study and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:387–396. doi: 10.1002/ajmg.b.30991. [DOI] [PubMed] [Google Scholar]
- 63.Galfalvy H., Huang YY., Oquendo MA., Currier D., Mann JJ. Increased risk of suicide attempt in mood disorders and TPH1 genotype. J Affect Disord. 2009;115:331–338. doi: 10.1016/j.jad.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manegold C., Hoffmann GF., Degen I., et al. Aromatic L-amino acid decarboxylase deficiency: clinical features, drug therapy and follow-up. J Inherit Metab Dis. 2009;32:371–380. doi: 10.1007/s10545-009-1076-1. [DOI] [PubMed] [Google Scholar]
- 65.Sirek A., Sirek OV. Serotonin: a review. Can Med Assoc J . 1970;102:846–849. [PMC free article] [PubMed] [Google Scholar]
- 66.Nakatani Y., Sato-Suzuki I., Tsujino N., et al. Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur J Neurosci. 2008;27:2466–2472. doi: 10.1111/j.1460-9568.2008.06201.x. [DOI] [PubMed] [Google Scholar]
- 67.Coccaro EF., Lee R., Kavoussi RJ. Inverse relationship between numbers of 5-HT transporter binding sites and life history of aggression and intermittent explosive disorder. J Psychiatr Res. 2010;44:137–142. doi: 10.1016/j.jpsychires.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Coccaro EF., Lee R. Cerebrospinal fluid 5-hydroxyindolacetic acid and homovanillic acid: reciprocal relationships with impulsive aggression in human subjects. J Neural Transm. 2010;117:241–248. doi: 10.1007/s00702-009-0359-x. [DOI] [PubMed] [Google Scholar]
- 69.Asellus P., Nordstrom P., Jokinen J. Cholesterol and CSF 5-HIAA in attempted suicide. J Affect Disord. 2010;125:388–392. doi: 10.1016/j.jad.2010.02.111. [DOI] [PubMed] [Google Scholar]
- 70.Jokinen J., Nordstrom AL., Nordstrom P. Cholesterol, CSF 5-HIAA, violence and intent in suicidal men. Psychiatry Res. 2010;178:217–219. doi: 10.1016/j.psychres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 71.Wallner B., Machatschke IH. The evolution of violence in men: the function of central cholesterol and serotonin. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:391–397. doi: 10.1016/j.pnpbp.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Hardisty RM., Stacey RS. 5-Hydroxytryptamine in normal human platelets. J Physiol. 1955;130:711–720. doi: 10.1113/jphysiol.1955.sp005437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leger L., Mouren-Mathieu AM., Descarries L. [Radioautographic identification of central monoaminergic neurons by local micro-instillation of tritiated serotonin or noradrenaline in cats]. C R Acad Sci Hebd Seances Acad Sci D. 1978;286:1523–1526. [PubMed] [Google Scholar]
- 74.Lichtensteiger W., Langemann H. The uptake of serotonin by central neurons normally containing catecholamines. Adv Pharmacol. 1968;6(Pt A):123. doi: 10.1016/s1054-3589(08)61163-1. [DOI] [PubMed] [Google Scholar]
- 75.VonWartburg JP. Uptake of 5-hydroxytryptophan-3-C14 and its metabolism in rat brain cortex slices. Can J Biochem Physiol. 1962;40:1439–1448. [PubMed] [Google Scholar]
- 76.Apparsundaram S., Stockdale DJ., Henningsen RA., Milla ME., Martin RS. Antidepressants targeting the serotonin reuptake transporter act via a competitive mechanism. J Pharmacol Exp Ther. 2008;327:982–990. doi: 10.1124/jpet.108.142315. [DOI] [PubMed] [Google Scholar]
- 77.Steiner JA., Carneiro AM., Wright J., et al. cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake. Mol Brain. 2009;2:26. doi: 10.1186/1756-6606-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramamoorthy S., Bauman AL., Moore KR., et al. Antidepressant - and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffman BJ., Mezey E., Brownstein MJ. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- 80.Demchyshyn LL., Pristupa ZB., Sugamori KS., et al. Cloning, expression, and localization of a chloride-facilitated, cocaine-sensitive serotonin transporter from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1994;91:5158–5162. doi: 10.1073/pnas.91.11.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amilhon B., Lepicard E., Renoir T., et al. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Austin MC., Bradley CC., Mann JJ., Blakely RD. Expression of serotonin transporter messenger RNA in the human brain. J Neurochem. 1994;62:2362–2367. doi: 10.1046/j.1471-4159.1994.62062362.x. [DOI] [PubMed] [Google Scholar]
- 83.Harro J., Merenakk L., Nordquist N., Konstabel K., Comasco E., Oreland L. Personality and the serotonin transporter gene: Associations in a longitudinal population-based study. Biol Psychol. 2009;81:9–13. doi: 10.1016/j.biopsycho.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Nordquist N., Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders--a review. Ups J Med Sci. 2010;115:2–10. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marini S., Bagnoli S., Bessi V., et al. Implication of serotonin-transporter (5-HTT) gene polymorphism in subjective memory complaints and mild cognitive impairment (MCI). Arch Gerontol Geriatr. 2010:In press. doi: 10.1016/j.archger.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 86.Steiner JA., Carneiro AM., Blakely RD. Going with the flow: traffickingdependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willeit M., Praschak-Rieder N. Imaging the effects of genetic polymorphisms on radioligand binding in the living human brain: a review on genetic neuroreceptor imaging of monoaminergic systems in psychiatry. Neuroimage. 2010;53:878–892. doi: 10.1016/j.neuroimage.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 88.Hendricks TJ., Fyodorov DV., Wegman LJ., et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 89.Kriegebaum CB., Gutknecht L., Bartke L., et al. The expression of the transcription factor FEV in adult human brain and its association with affective disorders. J Neural Transm. 2010;117:831–836. doi: 10.1007/s00702-010-0405-8. [DOI] [PubMed] [Google Scholar]
- 90.Baudry A., Mouillet-Richard S., Schneider B., Launay JM., Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 91.Zhang YW., Gesmonde J., Ramamoorthy S., Rudnick G. Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J Neurosci. 2007;27:10878–10886. doi: 10.1523/JNEUROSCI.0034-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christiansen L., Tan Q., Iachina M., et al. Candidate gene polymorphisms in the serotonergic pathway: influence on depression symptomatology in an elderly population. Biol Psychiatry. 2007;61:223–230. doi: 10.1016/j.biopsych.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 93.Glatt CE., Wahner AD., White DJ., Ruiz-Linares A., Ritz B. Gain-offunction haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006;15:299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Z., Walther D., Yu XY., Li S., Drgon T., Uhl GR. SLC18A2 promoter haplotypes and identification of a novel protective factor against alcoholism. Hum Mol Genet. 2005;14:1393–1404. doi: 10.1093/hmg/ddi148. [DOI] [PubMed] [Google Scholar]
- 95.Gaddum JH., Picarelli ZP. Two kinds of tryptamine receptor. Br J Pharmacol Chemother. 1957;12:323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peroutka SJ., Snyder SH. Two distinct serotonin receptors: regional variations in receptor binding in mammalian brain. Brain Res. 1981;208:339–347. doi: 10.1016/0006-8993(81)90562-x. [DOI] [PubMed] [Google Scholar]
- 97.Gothert M., Schlicker E. Classification of serotonin receptors. J Cardiovasc Pharmacol. 1987;10 (suppl 3):S3–S7. [PubMed] [Google Scholar]
- 98.Hannon J., Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 99.Barnes NM., Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 100.Fink KB., Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- 101.Beattie DT., Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:181–203. doi: 10.1007/s00210-008-0276-9. [DOI] [PubMed] [Google Scholar]
- 102.Sanger GJ. 5-Hydroxytryptamine and the gastrointestinal tract: where next? Trends Pharmacol Sci. 2008;29:465–471. doi: 10.1016/j.tips.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 103.Barnes NM., Hales TG., Lummis SC., Peters JA. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holbrook JD., Gill CH., Zebda N., et al. Characterisation of 5-HT3C, 5HT3D and 5-HT3E receptor subunits: evolution, distribution and function. J Neurochem. 2009;108:384–396. doi: 10.1111/j.1471-4159.2008.05775.x. [DOI] [PubMed] [Google Scholar]
- 105.Bockaert J., Perroy J., Becamel C., Marin P., Fagni L. GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annu Rev Pharmacol Toxicol. 2010;50:89–109. doi: 10.1146/annurev.pharmtox.010909.105705. [DOI] [PubMed] [Google Scholar]
- 106.Millan MJ., Marin P., Bockaert J., la Cour CM. Signaling at G-proteincoupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 107.arin T., Saettel N., Villain J., et al. 3D Pharmacophore, hierarchical methods, and 5-HT4 receptor binding data. J Enzyme Inhib Med Chem. 2008;23:593–603. doi: 10.1080/14756360802204748. [DOI] [PubMed] [Google Scholar]
- 108.Bhattacharyya S., Puri S., Miledi R., Panicker MM. Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase Cmediated mechanisms. Proc Natl Acad Sci U S A. 2002;99:14470–14475. doi: 10.1073/pnas.212517999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Idkowiak-Baldys J., Baldys A., Raymond JR., Hannun YA. Sustained receptor stimulation leads to sequestration of recycling endosomes in a classical protein kinase C-and phospholipase D-dependent manner. J Biol Chem. 2009;284:22322–22331. doi: 10.1074/jbc.M109.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Erritzoe D., Holst K., Frokjaer VG., et al. A nonlinear relationship between cerebral serotonin transporter and 5-HT(2A) receptor binding: an in vivo molecular imaging study in humans. J Neurosci. 2010;30:3391–3397. doi: 10.1523/JNEUROSCI.2852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corradi J., Gumilar F., Bouzat C. Single-channel kinetic analysis for activation and desensitization of homomeric 5-HTA receptors. Biophys J. 2009;97:1335–1345. doi: 10.1016/j.bpj.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gonzalez-Maeso J., Ang RL., Yuen T., et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruno A., Guadix AE., Costantino G. Molecular dynamics simulation of the heterodimeric mGluR2/5HT(2A) complex. An atomistic resolution study of a potential new target in psychiatric conditions. J Chem Inf Model. 2009;49:1602–1616. doi: 10.1021/ci900067g. [DOI] [PubMed] [Google Scholar]
- 114.Gonzalez-Maeso J. Anxious interactions. Nat Neurosci. 2010;13:524–526. doi: 10.1038/nn0510-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Magalhaes AC., Holmes KD., Dale LB., et al. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci. 2010;13:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allen JA., Yadav PN., Roth BL. Insights into the regulation of 5-HT2A serotonin receptors by scaffolding proteins and kinases. Neuropharmacology. 2008;55:961–968. doi: 10.1016/j.neuropharm.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zimmer L., Riad M., Rbah L., et al. Toward brain imaging of serotonin 5HT1A autoreceptor internalization. Neuroimage. 2004;22:1421–1426. doi: 10.1016/j.neuroimage.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 118.Joyce D., Summerfield A. Effects of drugs related to serotonin on water intake and defecation in rats. Implications for behavioural studies. Arch Int Pharmacodyn Ther. 1966;161:489–494. [PubMed] [Google Scholar]
- 119.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 120.Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat. Cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 121.Leger L., Charnay Y., Hof PR., Bouras C., Cespuglio R. Anatomical distribution of serotonin-containing neurons and axons in the central nervous system of the cat. J Comp Neurol. 2001;433:157–182. [PubMed] [Google Scholar]
- 122.Azmitia EC., Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv Neurol. 1986;43:407–468. [PubMed] [Google Scholar]
- 123.Nobin A., Bjorklund A. Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand Suppl. 1973;388:1–40. [PubMed] [Google Scholar]
- 124.Takahashi H., Nakashima S., Ohama E., Takeda S., Ikuta F. Distribution of serotonin-containing cell bodies in the brainstem of the human fetus determined with immunohistochemistry using antiserotonin serum. Brain Dev. 1986;8:355–365. doi: 10.1016/s0387-7604(86)80055-9. [DOI] [PubMed] [Google Scholar]
- 125.Baker KG., Halliday GM., Halasz P., et al. Cytoarchitecture of serotoninsynthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991;7:301–320. doi: 10.1002/syn.890070407. [DOI] [PubMed] [Google Scholar]
- 126.Baker KG., Halliday GM., Hornung JP., Geffen LB., Cotton RG., Tork I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience. 1991;42:757–775. doi: 10.1016/0306-4522(91)90043-n. [DOI] [PubMed] [Google Scholar]
- 127.Halliday GM., Li YW., Joh TH., et al. Distribution of monoamine-synthesizing neurons in the human medulla oblongata. J Comp Neurol. 1988;273:301–317. doi: 10.1002/cne.902730303. [DOI] [PubMed] [Google Scholar]
- 128.Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 129.Nieuwenhuis R. Monoamines: Chemoarchitecture of the Brain. Berlin, Germany: Springer Verlag;1985:33–41. [Google Scholar]
- 130.Kosofsky BE., Molliver ME. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- 131.O'Hearn E., Battaglia G., De Souza EB., Kuhar MJ., Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci. 1988;8:2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kojima M., Takeuchi Y., Goto M., Sano Y. Immunohistochemical study on the localization of serotonin fibers and terminals in the spinal cord of the monkey (Macaca fuscata). Cell Tissue Res. 1983;229:23–36. doi: 10.1007/BF00217878. [DOI] [PubMed] [Google Scholar]
- 133.Anderson IM., McKie. S., Elliott R., Williams SR., Deakin JF. Assessing human 5-HT function in vivo with pharmacoMRI. Neuropharmacology. 2008;55:1029–1037. doi: 10.1016/j.neuropharm.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 134.Freitas-Ferrari MC., Hallak JEC., Trzesniak C., et al. Neuroimaging in social anxiety disorder: a systematic review of the litterature. Progress Psychopharmacol Biol Psy. 2010;34:565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 135.Murphy SE. Using functional neuroimaging to investigate the mechanisms of action of selective serotonin reuptake inhibitors (SSRIs). Curr Pharm Des. 2010;16:1990–1997. doi: 10.2174/138161210791293051. [DOI] [PubMed] [Google Scholar]
- 136.Huang Y., Zheng MQ., Gerdes JM. Development of effective PET and SPECT imaging agents for the serotonin transporter: has a twenty-year journey reached its destination?. Curr Top Med Chem. 2010;10:1499–1526. doi: 10.2174/156802610793176792. [DOI] [PubMed] [Google Scholar]
- 137.Smith DF., Jakobsen S. Molecular tools for assessing human depression by positron emission tomography. Eur Neuropsychopharmacol. 2009;19:611–628. doi: 10.1016/j.euroneuro.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 138.Gallezot JD., Nabulsi N., Neumeister A., et al. Kinetic modeling of the serotonin 5-HT(1B) receptor radioligand [C]P943 in humans. J Cereb Blood Flow Metab. 2010;30:196–210. doi: 10.1038/jcbfm.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marner L., Gillings N., Madsen K., et al. Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. Neuroimage. 2010;50:855–861. doi: 10.1016/j.neuroimage.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 140.arnas K., Nyberg S., Halldin C., et al. Quantitative analysis of [C]AZ10419369 binding to 5-HT(1B) receptors in human brain. J Cereb Blood Flow Metab. 2010:In press. doi: 10.1038/jcbfm.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pike VW. PET radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol Sci. 2009;30:431–440. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fink M., Wadsak W., Savli M., et al. Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. Neuroimage. 2009;45:598–605. doi: 10.1016/j.neuroimage.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 143.Witte AV., Floel A., Stein P., et al. Aggression is related to frontal serotonin-1A receptor distribution as revealed by PET in healthy subjects. Hum Brain Mapp. 2009;30:2558–2570. doi: 10.1002/hbm.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Erritzoe D., Frokjaer VG., Haahr MT., et al. Cerebral serotonin transporter binding is inversely related to body mass index. Neuroimage. 2010;52:284–289. doi: 10.1016/j.neuroimage.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 145.Kupers R., Frokjaer VG., Naert A., et al. A PET [18F]altanserin study of 5HT2A receptor binding in the human brain and responses to painful heat stimulation. Neuroimage. 2009;44:1001–1007. doi: 10.1016/j.neuroimage.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 146.Kish SJ., Lerch J., Furukawa Y., et al. Decreased cerebral cortical serotonin transporter binding in ecstasy users: a positron emission tomography/[C]DASB and structural brain imaging study. Brain. 2010;133(Pt 6):1779–1797. doi: 10.1093/brain/awq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Borg J. Molecular imaging of the 5-HT(1A) receptor in relation to human cognition. Behav Brain Res. 2008;195:103–111. doi: 10.1016/j.bbr.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 148.Fakra E., Hyde LW., Gorka A., et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hariri AR., Drabant EM., Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 150.Munafo MR., Brown SM., Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoshida M., Takayanagi Y., Inoue K., et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Di Giovanni G., Esposito E., Di Matteo V. Role of serotonin in central dopamine dysfunction. CNS Neurosci. Ther. 2010;16:179–194. doi: 10.1111/j.1755-5949.2010.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Di Matteo V., Pierucci M., Esposito E., Crescimanno G., Benigno A., Di Giovanni G. Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. Prog Brain Res. 2008;172:423–463. doi: 10.1016/S0079-6123(08)00921-7. [DOI] [PubMed] [Google Scholar]
- 154.Haj-Dahmane S., Shen RY. Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J Pharmacol Exp Ther. 2009;331:186–196. doi: 10.1124/jpet.109.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Monti JM. The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep Med Rev. 2010;14:319–327. doi: 10.1016/j.smrv.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 156.Zoli M., Jansson A., Sykova E., Agnati LF., Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]
- 157.Griessenauer CJ., Chang SY., Tye SJ., et al. Wireless Instantaneous Neurotransmitter Concentration System: electrochemical monitoring of serotonin using fast-scan cyclic voltammetry-a proof-of-principle study. J Neurosurg. 2010;113:656–665. doi: 10.3171/2010.3.JNS091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pessoa L. Emotion and cognition and the amygdala: from "what is it?" to "what's to be done?". Neuropsychologia. 2010;48:3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Smith GS., Kramer E., Hermann C., et al. Serotonin modulation of cerebral glucose metabolism in depressed older adults. Biol Psychiatry. 2009;66:259–266. doi: 10.1016/j.biopsych.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fisher PM., Meltzer CC., Price JC., et al. Medial prefrontal cortex 5HT(2A) density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex. 2009;19:2499–2507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hariri AR., Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 162.Michelsen KA., Prickaerts J., Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer's disease. Prog Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- 163.Monti JM. The role of dorsal raphe nucleus serotonergic and nonserotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 2010;14:319–327. doi: 10.1016/j.smrv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 164.Lanfumey L., Mongeau R., Cohen-Salmon C., Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 165.Moser U., Wadsak W., Spindelegger C., et al. Hypothalamic serotonin1 A receptor binding measured by PET predicts the plasma level of dehydroepiandrosterone sulfate in healthy women. Neurosci Lett. 2010;476:161–165. doi: 10.1016/j.neulet.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 166.Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 167.Bjork K., Sjogren B., Svenningsson P. Regulation of serotonin receptor function in the nervous system by lipid rafts and adaptor proteins. Exp Cell Res. 2010;316:1351–1356. doi: 10.1016/j.yexcr.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 168.Benmansour S., Deltheil T., Piotrowski J., et al. Influence of brainderived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587:90–98. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 169.Savelieva KV., Zhao S., Pogorelov VM., et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Herzog H., Pietrzyk U., Shah NJ., Ziemons K. The current state, challenges and perspectives of MR-PET. Neuroimage. 49:2072–2082. doi: 10.1016/j.neuroimage.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 171.King MV., Marsden CA., Fone KC. A role for the 5-HT(1A), 5-HT4 and 5HT6 receptors in learning and memory. Trends Pharmacol Sci. 2008;29:482–492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 172.Mostany R., Pazos A., Castro ME. Autoradiographic characterisation of [35S]GTPgammaS binding stimulation mediated by 5-HT1B receptor in postmortem human brain. Neuropharmacology. 2005;48:25–33. doi: 10.1016/j.neuropharm.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 173.Varnas K., Hall H., Bonaventure P., Sedvall G. Autoradiographic mapping of 5-HT(1B) and 5-HT(1D) receptors in the post mortem human brain using [H]GR 125743. Brain Res. 2001;915:47–57. doi: 10.1016/s0006-8993(01)02823-2. [DOI] [PubMed] [Google Scholar]
- 174.Bai F., Yin T., Johnstone EM., et al. Molecular cloning and pharmacological characterization of the guinea pig 5-HT1E receptor. Eur J Pharmacol. 2004;484:127–139. doi: 10.1016/j.ejphar.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 175.Lucaites VL., Krushinski JH., Schaus JM., Audia JE., Nelson DL. [3H]LY334370, a novel radioligand for the 5-HT1F receptor. II. Autoradiographic localization in rat, guinea pig, monkey and human brain. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:178–184. doi: 10.1007/s00210-005-1036-8. [DOI] [PubMed] [Google Scholar]
- 176.Meyer PT., Bhagwagar Z., Cowen PJ., Cunningham VJ., Grasby PM., Hinz R. Simplified quantification of 5-HT2A receptors in the human brain with [11C]MDL 100,907 PET and non-invasive kinetic analyses. Neuroimage. 2010;50:984–993. doi: 10.1016/j.neuroimage.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 177.Bonhaus DW., Bach C., DeSouza A., et al. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Giorgetti M., Tecott LH. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur J Pharmacol. 2004;488:1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 179.Varnas K., Halldin C., Pike VW., Hall H. Distribution of 5-HT4 receptors in the postmortem human brain--an autoradiographic study using [125I]SB 207710. Eur Neuropsychopharmacol. 2003;13:228–234. doi: 10.1016/s0924-977x(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 180.Lucas G., Rymar VV., Du J., et al. Serotonin 4 (5-HT4) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55:712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 181.Thomas DR. 5-ht5A receptors as a therapeutic target. Pharmacol Ther. 2006;111:707–714. doi: 10.1016/j.pharmthera.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 182.Upton N., Chuang TT., Hunter AJ., Virley DJ. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer's disease. Neurotherapeutics. 2008;5:458–469. doi: 10.1016/j.nurt.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl). 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]