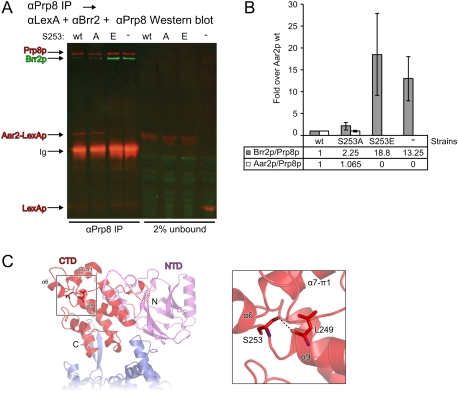
Figure 6.
Influence of Aar2p and variants on the Brr2p–Prp8p interaction. (A,B) Prp8p interacts with more Brr2p when bound by less Aar2p (S253E). (A) Full-length Prp8p was immunoprecipitated from yeast cell extracts containing the highly expressed wild-type, S253A, or S253E variants of Aar2-LexAp, or with LexAp as a control; blotted; and probed with rat anti-Brr2, rabbit anti-LexA, and rabbit anti-Prp8 primary antibodies, followed by goat anti-rat IRDye 680LT and donkey anti-rabbit IRDye 800CW secondary antibodies. The results were visualized and quantified using a LI-COR Odyssey imaging system. (B) Average ratios of precipitated Brr2p/Prp8p and Aar2p/Prp8p are shown for two experiments. Error bars indicate the maximum and minimum values obtained. A similar analysis was also performed in triplicate using detection by standard Western blot (see Supplemental Fig. S5F). (C) Positioning of S253 in the Aar2pSub–Prp8pRNaseH complex structure. (Left) Structural overview. The region surrounding Aar2p S253 is boxed. Protein termini, domains, and selected secondary structure elements are labeled. (Right) Closeup view of the boxed region. Selected residues are shown as sticks and colored by atom type as in Figure 3C. (Dashed line) Hydrogen bond.