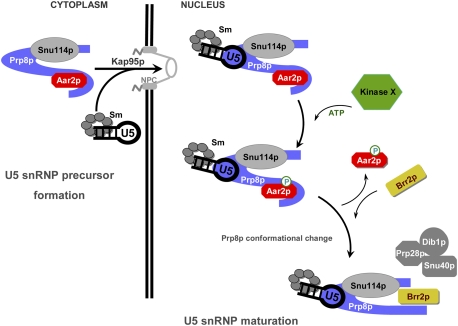
Figure 7.
Model for the U5 snRNP maturation. New elements added to the U5 snRNP maturation model proposed by Boon et al. (2007). Aar2p-U5 snRNP is assembled in the cytoplasm and transported to the nucleus. In the nucleus, Aar2p is phosphorylated by an unidentified kinase (Kinase X). Phosphorylated Aar2p exhibits reduced affinity for Prp8p, leaves the pre-U5 snRNP, and allows Brr2p entry. In view of our finding that the RNaseH and Jab1/MPN domains, which are joined by a flexible linker region, do not interact, we propose that the C terminus of Prp8p takes on a different conformation when not bound by Aar2p. Additional U5 snRNP proteins lacking from Aar2p-U5 snRNP are presumably assembled concomitantly with Brr2p (Boon et al. 2007). Phosphorylated Aar2p has a possible active role in recruiting Brr2p (not shown).