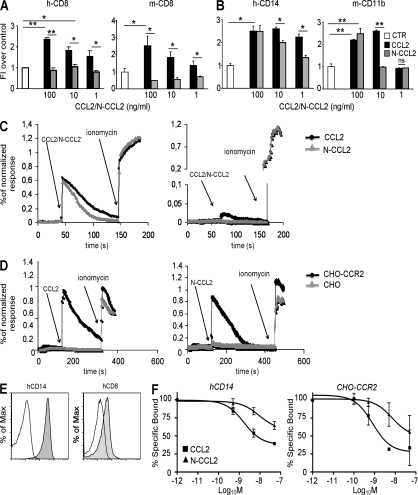
Figure 3.
RNS alter the biological activity of human and mouse CCL2. (A and B) Human or mouse CD8+ T lymphocytes (A) and human CD14+ or mouse CD11b+ myeloid cells (B) were exposed to a gradient of recombinant human or mouse CCL2 (100, 10, or 1 ng/ml). Chemokines were either untreated (CCL2) or RNS treated (N-CCL2). Transmigrated cells were counted, and the results were expressed as fold induction over control. Data are representative of three different experiments and are expressed as the means ± SE. *, P ≤ 0.05; **, P ≤ 0.01. (C and D) Fluo-4/Fura-Red–loaded human T cells (CD14+ or CD8+; A) or CHO cells that were or were not expressing human CCR2 (CHO or CHO-CCR2; D) were stimulated with either CCL2 or N-CCL2 and free [Ca2+]i was measured by flow cytometry. Ionomycin was used as a positive control for the maximal Ca2+ influx. Data are representative of one out of three experiments. (E) Human CD8+ and CD14+ cells were stained with either anti–human CCR2 phycoerythrin-conjugated mAb or with its isotypic control. Cell fluorescence was analyzed by flow cytometry. The mean fluorescence intensity, normalized to isotype control staining, was 13.2 ± 1.7 and 2.3 ± 0.3 for hCD14 and hCD8 cells, respectively (P ≤ 0.05). (F) Competitive binding was performed by incubating CD14+ cells or CHO-CCR2 cells with 125I-hCCL2 in the presence of various concentrations of unlabeled, untreated (▪), or peroxynitrite-treated (Δ) hCCL2. After incubation, the cell-associated radioactivity was measured. Data are representative of three different experiments and are expressed as the means ± SE.