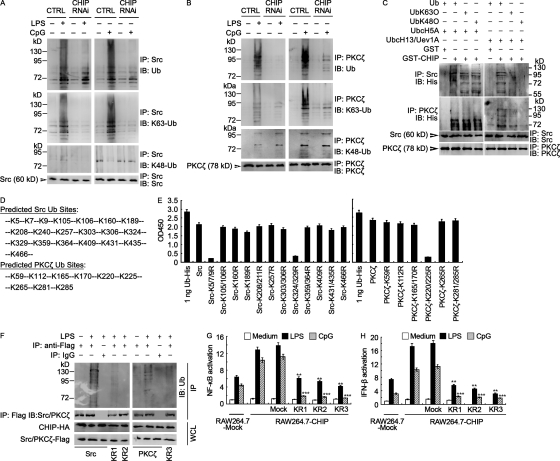
Figure 9.
CHIP polyubiquitinates Src and PKCζ. (A and B) In vivo polyubiquitination assays. RAW264.7 cells stably transfected with scrambled control RNAi (CTRL) or CHIP-specific RNAi (RNAi) were treated with 100 ng/ml LPS or 1 µM CpG ODN for 30 min. Src and PKCζ contained in lysates were then subjected to heat denaturing in 1% SDS and immunoprecipitated (IP) with Src (A) or PKCζ (B) antibody plus protein A/G agarose. Ubiquitination was examined by immunoblot (IB) using anti-ubiquitin (Ub), anti–K63-Ub, or anti–K48-Ub antibody as indicated. (C) In vitro polyubiquitination assays. GST-CHIP was incubated with recombinant Src or PKCζ in the presence of E1, His6-Ub derivatives, and UbcH5A or Ubc13/Uev1A E2/Ubc as indicated. Src and PKCζ were immunoprecipitated (IP) with anti-Src or anti-PKCζ antibody after heat denaturing in 1% SDS. UbK63O, ubiquitin containing only Lys63; UbK48O, ubiquitin containing only Lys48. (D) Predicted ubiquitination sites in Src and PKCζ using the Bayesian discriminant method-prediction of ubiquitination sites algorithm, as well as protein sequence alignment. (E) RAW264.7 cells were transiently transfected with Flag-tagged wild-type or mutant Src (left) or PKCζ (right) constructs for 48 h, and then treated with 100 ng/ml LPS for 30 min. Ubiquitin levels associated with Src/PKCζ (mutants) were evaluated by ELISA at 450 nm. 1 ng recombinant ubiquitin-His was used as positive control. (F) RAW264.7 cells stably transfected with CHIP-HA were transiently transfected with Flag-tagged wild-type or mutant (KR1-3) Src or PKCζ constructs for 48 h, and then treated with 100 ng/ml LPS for 30 min. Src and PKCζ contained in lysates were immunoprecipitated (IP) with Flag agarose or IgG plus protein A/G agarose as indicated. Ubiquitination was examined by immunoblot (IB) using anti-Ub antibody. (G and H) RAW264.7 cells stably transfected with mock vector (RAW264.7-Mock) or CHIP-HA vector (RAW264.7-CHIP) were transiently transfected with Flag-tagged Src and PKCζ mutants (KR1-3) and NF-κB (G) or IFN-β (H) reporters for 48 h, and then treated with 100 ng/ml LPS or 1 µM CpG ODN for 4 h. Data are shown as mean ± SD of triplicate samples. **, P < 0.01; ***, P < 0.001 (as compared with corresponding RAW264.7-CHIP cells transiently transfected with mock vector and treated with LPS/CpG). In F–H, KR1 indicates for Src-K5/7/9R, KR2 for Src-K324/329R, and KR3 for PKCζ-K220/225R. The data shown in A–C and E–H correspond to a representative experiment out of three performed.