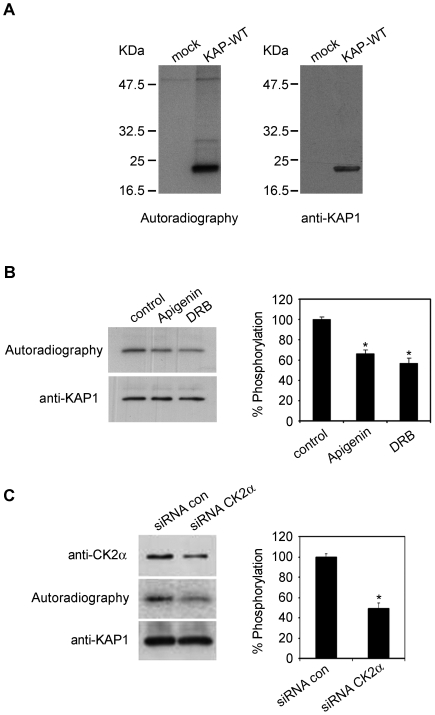
Figure 4. Protein kinase CK2 is involved in KAP phosphorylation in cultured cells.
(A) Transient transfected PCT3 cells with an empty vector (mock) or containing the KAP-WT construct were metabolically labeled with [32P]orthophosphate. Cell lysates were immunoprecipitated with the anti-HA antibody and the products resolved in an SDS-PAGE, transferred to a PVDF membrane and exposed to an X-ray film (left panel). Western blot with specific anti-KAP-1 antibodies demonstrates that the phosphorylated protein was KAP (right panel). (B) KAP-WT transfected PCT3 cells were exposed to the CK2 inhibitors apigenin and DRB for one hour before exposure of the cells to [32P]orthophosphate and for the ensuing three hours of metabolic labeling. Densitometric autoradiographic values were referred to values of Western blot assays performed with an anti-KAP antibody (left panel). The percentage of phosphorylation inhibition obtained in the presence of apigenin and DRB, in relation to the control situation, is graphically represented (right panel). *Significantly lower than control (Student-t test, p<0,05). (C) PCT3 cells were co-transfected with KAP-WT and either non-silencing control (siRNA con) or anti-CK2α siRNA. After 48h of transfection, the cells were exposed to [32P]orthophosphate, labeled for 3h and processed as indicated in (A). *Significantly lower than siRNA control (Student-t test, p<0,05).