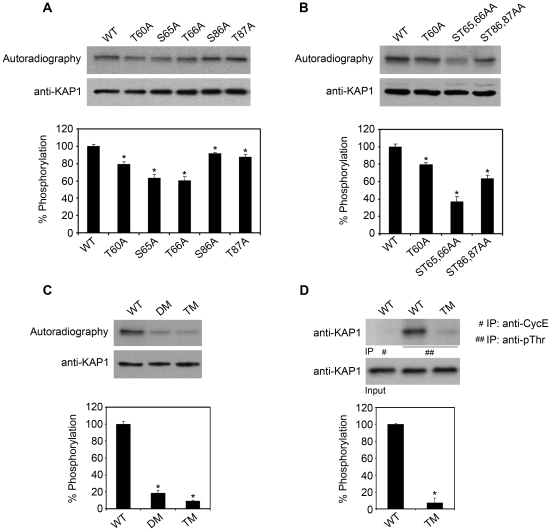
Figure 6. In cultured kidney cells KAP, is phosphorylated at residues located in the PEST sequence.
(A) Residues Thr60, Ser65, Thr66, Ser86 and Thr87 were mutated and KAP phosphorylation analyzed in 32P-labeled PCT3 cells overexpressing KAP mutants. Numbers in the lower panel of Fig. A and in the following panels were calculated by densitometric analyses of phosphorylated protein and normalized by total KAP protein levels. The ratio corresponding to KAP-WT protein was used as a reference in each case. * p<0,05 vs WT (Student-t test). (B) Individual domains that include T60A, ST65/66AA and ST86/87AA were also tested. As depicted in Fig. B, all three constructs resulted in a reduction in KAP phosphorylation. * p<0,05 vs WT (Student-t test). (C) New mutants that include T60A and ST65/66AA (KAP-DM) and residues T60A, ST65/66AA and ST86/87AA (KAP-TM) were also produced. The phosphorylation capacities of DM and TM constructs were assessed as explained in part A of this figure. * p<0,0001 vs WT (Student-t test).(D) Immunoprecipitation of KAP-WT and KAP-TM in transfected PCT3 cell extracts using anti-phospho-Thr antibodies (##), and further immunoblotting with anti-KAP specific antibodies showed that the Thr residues mutated in the KAP-TM form are phosphorylated in the WT form. Immunoprecipitation of KAP-WT cell extracts using anti-CycE antibodies was used as a negative control (#).* p<0,0001 vs WT (Student-t test).