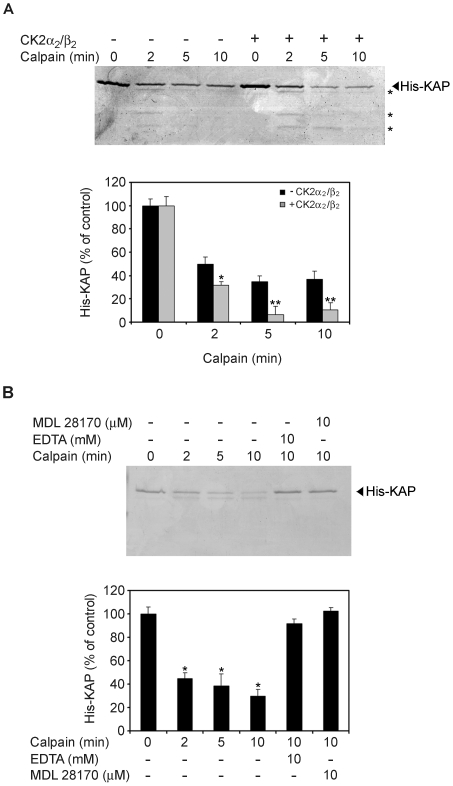
Figure 9. KAP degradation by calpain in vitro is enhanced by CK2 phosphorylation.
(A) CK2 phosphorylation promotes in vitro degradation of KAP by calpain. 0.5 µg of mouse recombinant His-KAP-purified protein were incubated in the presence (lanes 5 to 8) or absence (lanes 1 to 4) of 2 pmols of CK2α2/β2 and ATP for 30 min. To each reaction, 0.18 U of m-calpain were added and incubated in assay buffer containing 5 mM CaCl2 for 0, 2, 5 and 10 min. Reactions were stopped by addition of loading buffer and products run in a 15% SDS-PAGE. Proteins were visualized by Coomassie Blue staining and graphic representation after densitometric analysis shown in the lower panel of the figure. It represents the normalized signal quantifications, taking as 100% the densitometric values detected at time 0. * p<0,05 and ** p<0,01 vs time-paired His-KAP incubated in the absence of CK2α2/β2 (Student-t test). (B) Non-phosphorylated KAP degradation is due to calpain. 0.5 µg of mouse recombinant His-KAP-purified protein were incubated with 0.18 U of m-calpain in assay buffer containing 5 mM CaCl2, at different times; in these same conditions, at the 10 min time, 10 mM EDTA (lane 5) or 10 µM of MDL 28170 (lane 6) were also added. Degradation was visualized by Coomassie Blue in SDS-PAGE and quantification assessed by densitometric analysis (lower panel). * p<0,001 vs untreated His-KAP (Student-t test).