Abstract
Objective
Obstructive sleep apnea syndrome (OSAS) has been associated with cardiovascular complications and insulin resistance has been implicated in the pathogenesis and progression of atherosclerosis. We investigated whether insulin resistance is associated with OSAS independent of obesity.
Methods
A total of 183 male patients with OSAS and 52 healthy controls were assessed by nocturnal polysomnography (NPSG). After NPSG, serum concentrations of total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, glucose and insulin were measured. Insulin resistance was determined by calculating the homeostasis model assessment for insulin resistance (HOMA-IR).
Results
Subjects were divided into normal control, mild-to-moderate OSA group (n=96) and severe OSA group (n=87). There were no significant differences among groups in age, body mass index (BMI), neck circumference or waist circumference. Serum concentrations of total cholesterol, LDL cholesterol, triglycerides, glucose, insulin and HOMA-IR scores of normal controls did not differ from those of the mild-to-moderate or severe OSAS groups. HOMA-IR significantly correlated with anthropometric variables, oxygen desaturation index, triglyceride and LDL cholesterol. Stepwise multiple linear regression analysis showed that waist circumference (β=0.35) and triglycerides (β=0.27) were significant determinants of HOMA-IR (adjusted R2=20%, p<0.01).
Conclusion
Insulin resistance was related to obesity itself rather than OSAS severity or nocturnal hypoxemia-related variables. In preventing cardiovascular complications in OSAS patients, weight reduction should be considered.
Keywords: Obstructive sleep apnea syndrome, Insulin resistance, Obesity, HOMA-IR
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is characterized by nocturnal hypoxemia and frequent arousals during sleep caused by repetitive nocturnal respiratory pauses. OSAS is associated with increased rates of cardiovascular complications, as shown by the high prevalence of OSAS in patients with stroke, transient ischemic attack, or coronary heart disease.1-3 Atherosclerosis may be responsible for the relationships between OSAS and these cardiovascular and cerebrovascular diseases.4
Insulin resistance is a condition of an impaired response to insulin and a decrease in insulin-mediated glucose disposal,5 and in the pre-diabetic state, it is associated with atherogenic changes as a mechanism leading to type II diabetes.6 Recently studies have implicated insulin resistance in the pathogenesis and progression of atherosclerosis7,8 as one component of metabolic syndrome which comprises insulin resistance, obesity, hypertension and dyslipidemia.9
Insulin resistance is closely associated with obesity,10 although this relationship is unclear in patients with OSAS. Although researchers have shown that either OSAS severity or degree of nocturnal hypoxemia is responsible for insulin resistance, independent of obesity,11-15 obesity itself rather than OSAS severity is reportedly of importance in insulin resistance.16-18 These conflicting results may be due to differences in the clinical characteristics of study patients or study methods. Because obesity is associated with the development of insulin resistance, adjusting for obesity is important when comparing degrees of insulin resistance. Several previous studies, however, did not control for anthropometric variables.11,12,14,17 We therefore investigated the relationship between insulin resistance and OSAS to identify the factors that may influence insulin resistance in patients with OSAS.
METHODS
Subjects
This study analyzed 183 male patients with OSAS who had been referred to the sleep laboratory at Seoul National University Bundang Hospital for nocturnal polysomnography (NPSG). Of these, 96 patients had mild to moderate OSAS [apnea hypopnea index (AHI) ≥5 and <30] and 87 patients had severe OSAS (AHI ≥30). We also included 52 normal healthy subjects with AHI <5 on the NPSG. Based on clinical interviews and medical records, we excluded subjects with inflammatory disease, chronic obstructive pulmonary disease (COPD), or cardiovascular diseases such as coronary artery disease, myocardial infarction or congestive heart failure. We also excluded patients taking antihypertensive, antihyperlipidemic, or hypoglycemic agents.19 The study protocol was approved by the Institutional Review Board of the Seoul National University Bundang Hospital, and all subjects provided written informed consents.
Polysomnography
Overnight polysomnography was performed using an Embla™ N 7000 recording system (Embla; Reykjavik, Iceland) and standard electrodes and sensors. Electroencephalography electrodes were applied at C3/A2, O1/A2 and O2/A1, and two electrooculography (EOG) electrodes were applied at the sides of both eyes. Submental electromyography (EMG) electrodes were applied to the submentalis muscle, and the EMGs of both anterior tibialis muscles recorded limb movements during sleep. Strain gauges were used to record chest and abdominal respiratory movements, and a nasal pressure cannula was used to record airflow. Arterial oxygen saturation was measured using pulse oximeters applied to index fingers. Based on the published criteria,20 we scored every epoch of 30-sec NPSG. Apnea was defined as the complete cessation of airflow for at least 10 seconds. Hypopnea was defined as a substantial (>50%) reduction in airflow for at least 10 seconds or a moderate reduction in airflow for at least 10 seconds associated with EEG arousals or oxygen desaturation (≥4%).21 AHI was defined as the total number of apnea and hypopnea episodes per hour of sleep and oxygen desaturation index (ODI) was calculated as the number of oxygen desaturations (≥4%) per hour of sleep.
Measurement of insulin resistance
On the morning after NPSG, blood was drawn from each subject, who had been fasting for at least 8 hours. Serum concentrations of total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, glucose and insulin were measured by standard methods. Insulin resistance was assessed by calculating homeostasis model assessment for insulin resistance (HOMA-IR) using the equation: HOMA-IR=fasting serum insulin (U/mL)×fasting plasma glucose (mmol/L)/22.5.
Statistical analysis
SPSS version 12.0K for Windows (SPSS Inc, Chicago, IL, USA) was used for all statistical analyses. Results are presented as means±SD. The Kolmogorov-Smirnov test was used to confirm normality. Differences in parametric clinical variables among the three groups were assessed by analysis of variance (ANOVA) with the Tukey posthoc test. The Kruskal-Wallis test was used to compare non-parametric variables. Pearson or Spearman correlation coefficients were calculated to determine relationships between insulin resistance and clinical or polysomnographic variables, and stepwise multiple regression analysis was used to identify factors that contributed to insulin resistance. Statistical significance was set at p<0.05 for two-tailed tests.
RESULTS
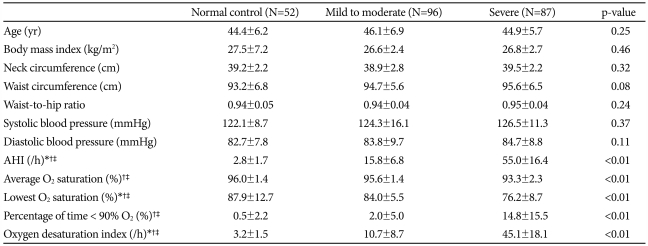
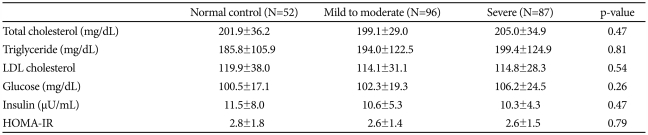
There were no significant differences among the three groups in age, blood pressure, or obesity-related variables; body mass index (BMI), neck circumference, waist circumference and waist-to-hip ratio (WHR) (Table 1). Mean AHI, percentage of time below 90% oxygen saturation, and ODI were significantly higher in the severe OSAS group than in the control and mild-to-moderate OSAS groups (p<0.01 for each), and average oxygen saturation and lowest oxygen saturation were significantly lower in the severe OSAS group. Serum concentrations of total cholesterol, LDL cholesterol, triglycerides, glucose and insulin of the control group did not differ from those of either the mild-to-moderate or the severe OSAS groups (Table 2). In addition, HOMA-IR did not differ significantly among the three groups.
Table 1.
Demographic and clinical characteristics of subjects
*p<0.05, normal controls vs. mild to moderate, †p<0.05, normal controls vs. severe, ‡p<0.05, mild to moderate vs. severe. AHI: apnea hypopnea index
Table 2.
Laboratory findings of subjects
HOMA-IR: homeostasis model assessment for estimating insulin resistance=[fasting serum insulin (U/mL)×fasting plasma glucose (mmol/L)/22.5], LDL: low-density lipoprotein
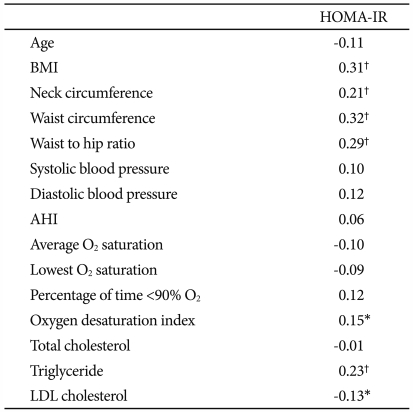
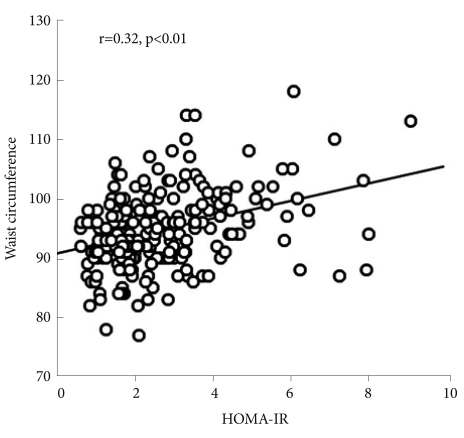
Table 3 shows the correlation coefficients of HOMA-IR with the demographic and polysomnographic variables. We found that HOMA-IR significantly correlated with BMI (r=0.31), neck circumference (r=0.21), waist circumference (r=0.32, Figure 1), and WHR (r=0.29) (p<0.01 for each). In addition, ODI (r=0.15, p=0.02), triglyceride (r=0.23, p<0.01), and LDL cholesterol (r=-0.13, p=0.04) significantly correlated with HOMA-IR. HOMA-IR, however, was not significantly correlated with AHI, average O2 saturation, lowest O2 saturation, or percentage of time below 90% O2 saturation. When we performed stepwise multiple linear regression analysis to identify factors significantly contributing to HOMA-IR, we found waist circumference (β=0.35) and triglyceride (β=0.27) to be significant independent determinants of HOMA-IR (adjusted R2=20%, p<0.01).
Table 3.
Correlation between HOMA-IR and demographic or clinical variables
*p-value<0.05, †p-value<0.01. HOMA-IR: homeostasis model assessment for estimating insulin resistance, BMI: body mass index, AHI: apnea hypopnea index, LDL: low-density lipoprotein
Figure 1.
Correlation between HOMA-IR and waist circumference. HOMA-IR: homeostasis model assessment for insulin resistance.
DISCUSSION
We show here that insulin resistance in patients with OSAS significantly correlated with several anthropometric variables (BMI, neck circumference, waist circumference and WHR), but not with AHI or any nocturnal hypoxemia-related variables except ODI. Although the correlations between HOMA-R and the anthropometric variables were modest, multivariate analysis still found waist circumference to be an independent predicator of HOMA-IR. These findings indicate that changes of insulin resistance in OSAS patients result from obesity rather than the severity of OSAS or nocturnal hypoxemia. In this study, we found trends of triglycerides (TG) increases and insulin level decreases in the following order; normal controls, mild-to-moderate OSAS and severe OSAS patients. These findings might be comparable to those in other studies, but no definite relation between either TG or insulin and OSAS could be assumed in this study due to the lack of statistical significance.
Although insulin resistance has been associated with OSAS,22 the factors determining insulin resistance in OSAS patients are unclear. Insulin resistance is related to obesity.10 Many patients with OSAS are obese, and studies have repeatedly shown obesity has an impact on the increased prevalence of OSAS.23,24 Researchers reported that obesity itself has been implicated in insulin resistance in OSAS patients.16-18 In contrast, other reports show that the severity of OSAS (AHI) or nocturnal hypoxemia, as determined by average O2 saturation or minimum O2 saturation, correlate with insulin resistance rather than obesity.11-15 In several previous studies, apneic patients were more obese than control subjects, as assessed by BMI11,12,14 or waist circumference.17 Those conflicting results regarding the actual contributing factors to insulin resistance may be due to differences in the clinical characteristics of study patients or study methods. Thus, anthropometric variables should be adjusted when comparing the degree of insulin resistance in patients with OSAS and this study has the merit of controlling obesity-related parameters between OSAS patients and normal controls.
We observed no significant differences in HOMA-IR among these groups of individuals categorized by severity of OSAS, but waist circumference was a significant determinant of HOMA-IR. Our results indicate that obesity itself, rather than severity of OSAS or nocturnal hypoxemia, may influence insulin resistance. In our study, there were no significant differences in anthropometric variables (BMI, neck circumference, waist circumference and WHR) among the normal control, mild-to-moderate OSAS, and severe OSAS groups. In addition, serum levels of cholesterol, tyiglyceride, LDL cholesterol, and glucose of the normal control also did not differ from those of mild-to-moderate or severe OSAS group. We controlled anthropometric and metabolic variables to eliminate confounding influences of those variables on the relationship between OSAS and insulin resistance. We also excluded patients with cardiovascular disorders, which could lead to a negative selection bias because this may have included only OSAS patients at lower risk for cardiovascular disorders. However, we sought for independent relationship between OSAS and insulin resistance, and it could be an acceptable approach to that purpose to control confounding variables as strictly as possible. Cardiovascular disorders and obesity have been known to correlate with insulin resistance. Thus we excluded patients with cardiovascular disorders and controlled for obesity between OSAS patients and normal controls.
The results of nasal continuous positive airway pressure (nCPAP) studies support our data. The nCPAP is regarded as the most effective therapy for OSAS. Insulin resistance, however, cannot be improved by nCPAP in the absence of significant changes in anthropometric profiles. Neither insulin resistance nor weight significantly changes after nCPAP treatment,25-32 and we have also reported that insulin resistance was not improved by at least 3 months of nCPAP treatment.33 These results indicate insulin resistance is associated with obesity rather than with severity of OSAS, although these conclusions are still conflicting with other studies.34-36
We included only male patients with OSAS because gender may affect insulin resistance.37 This, however, may limit the applicability of these results to general populations. Our OSAS patients had lower BMIs compared to subjects of studies in western countries. The low percentage of obese OSAS patients in our study may be a clinical characteristic of Asian patients.38 It may be argued that the sample size in this study was insufficient to detect any significant difference, but the number of subjects in this study (n=235) is comparable to numbers in previous studies.11,12,15 In conclusion, our findings show insulin resistance in OSAS patients is due to obesity rather than to severity of OSAS.
References
- 1.Hermann DM, Bassetti CL. Sleep-disordered breathing and stroke. Curr Opin Neurol. 2003;16:87–90. doi: 10.1097/01.wco.0000053587.70044.be. [DOI] [PubMed] [Google Scholar]
- 2.Mohsenin V. Sleep-related breathing disorders and risk of stroke. Stroke. 2001;32:1271–1278. doi: 10.1161/01.str.32.6.1271. [DOI] [PubMed] [Google Scholar]
- 3.Andreas S, Schulz R, Werner GS, Kreuzer H. Prevalence of obstructive sleep apnoea in patients with coronary artery disease. Coron Artery Dis. 1996;7:541–545. [PubMed] [Google Scholar]
- 4.Lévy P, Pépin JL, Arnaud C, Baguet JP, Dematteis M, Mach F. Obstructive sleep apnea and atherosclerosis. Prog Cardiovasc Dis. 2009;51:400–410. doi: 10.1016/j.pcad.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Kendall DM, Sobel BE, Coulston AM, Peters Harmel AL, McLean BK, Peragallo-Dittko V, et al. The insulin resistance syndrome and coronary artery disease. Coron Artery Dis. 2003;14:335–348. doi: 10.1097/01.mca.0000076512.29238.2a. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Mykkänen L, Festa A, Burke JP, Stern MP. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation. 2000;101:975–980. doi: 10.1161/01.cir.101.9.975. [DOI] [PubMed] [Google Scholar]
- 7.Sourij H, Schmoelzer I, Dittrich P, Paulweber B, Iglseder B, Wascher TC. Insulin resistance as a risk factor for carotid atherosclerosis: a comparison of the Homeostasis Model Assessment and the short insulin tolerance test. Stroke. 2008;39:1349–1351. doi: 10.1161/STROKEAHA.107.502799. [DOI] [PubMed] [Google Scholar]
- 8.Kernan WN, Inzucchi SE, Viscoli CM, Brass LM, Bravata DM, Horwitz RI. Insulin resistance and risk for stroke. Neurology. 2002;59:809–815. doi: 10.1212/wnl.59.6.809. [DOI] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 10.Ascaso JF, Romero P, Real JT, Lorente RI, Martínez-Valls J, Carmena R. Abdominal obesity, insulin resistance, and metabolic syndrome in a southern European population. Eur J Intern Med. 2003;14:101–106. doi: 10.1016/S0953-6205(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 11.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 12.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 13.Tassone F, Lanfranco F, Gianotti L, Pivetti S, Navone F, Rossetto R, et al. Obstructive sleep apnoea syndrome impairs insulin sensitivity independently of anthropometric variables. Clin Endocrinol (Oxf) 2003;59:374–379. doi: 10.1046/j.1365-2265.2003.01859.x. [DOI] [PubMed] [Google Scholar]
- 14.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 15.Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179:228–234. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med. 1996;154:170–174. doi: 10.1164/ajrccm.154.1.8680675. [DOI] [PubMed] [Google Scholar]
- 17.Kapsimalis F, Varouchakis G, Manousaki A, Daskas S, Nikita D, Kryger M, et al. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186:209–217. doi: 10.1007/s00408-008-9082-x. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8:12–17. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A. Antihypertensive drugs and reversing of endothelial dysfunction in hypertension. Curr Hypertens Rep. 2000;2:64–70. doi: 10.1007/s11906-000-0061-8. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service / Brain Research Institute; 1968. [Google Scholar]
- 21.Gould GA, Whyte KF, Rhind GB, Airlie MA, Catterall JR, Shapiro CM, et al. The sleep hypopnea syndrome. Am Rev Respir Dis. 1988;137:895–898. doi: 10.1164/ajrccm/137.4.895. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–224. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 24.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 25.Murri M, Alcázar-Ramírez J, Garrido-Sánchez L, Linde F, Alcaide J, Cardona F, et al. Oxidative stress and metabolic changes after continuous positive airway pressure treatment according to previous metabolic disorders in sleep apnea-hypopnea syndrome patients. Transl Res. 2009;154:111–121. doi: 10.1016/j.trsl.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Carneiro G, Togeiro SM, Ribeiro-Filho FF, Truksinas E, Ribeiro AB, Zanella MT, et al. Continuous positive airway pressure therapy improves hypoadiponectinemia in severe obese men with obstructive sleep apnea without changes in insulin resistance. Metab Syndr Relat Disord. 2009;7:537–542. doi: 10.1089/met.2009.0019. [DOI] [PubMed] [Google Scholar]
- 27.Steiropoulos P, Papanas N, Nena E, Tsara V, Fitili C, Tzouvelekis A, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with Obstructive Sleep Apnea Hypopnea Syndrome: does adherence to CPAP treatment improve glycemic control. Sleep Med. 2009;10:887–891. doi: 10.1016/j.sleep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Collins B, Basta M, et al. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest. 2008;38:585–595. doi: 10.1111/j.1365-2362.2008.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teramoto S, Yamaguchi Y, Yamamoto H, Hanaoka Y, Ishii M, Hibi S, et al. Cardiovascular and metabolic effects of CPAP in obese obstructive sleep apnoea patients. Eur Respir J. 2008;31:223–225. doi: 10.1183/09031936.00105707. [DOI] [PubMed] [Google Scholar]
- 30.Trenell MI, Ward JA, Yee BJ, Phillips CL, Kemp GJ, Grunstein RR, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–687. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 31.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 32.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung S, Yoon IY, Lee CH, Kim JW. The effects of nasal continuous positive airway pressure on vascular functions and serum cardiovascular risk factors in obstructive sleep apnea syndrome. Sleep Breath. 2010;15:71–76. doi: 10.1007/s11325-009-0323-x. [DOI] [PubMed] [Google Scholar]
- 34.de Lima AM, Franco CM, de Castro CM, Bezerra Ade A, Ataíde L, Jr, Halpern A. Effects of nasal continuous positive airway pressure treatment on oxidative stress and adiponectin levels in obese patients with obstructive sleep apnea. Respiration. 2010;79:370–376. doi: 10.1159/000227800. [DOI] [PubMed] [Google Scholar]
- 35.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–692. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 36.Patruno V, Aiolfi S, Costantino G, Murgia R, Selmi C, Malliani A, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest. 2007;131:1393–1399. doi: 10.1378/chest.06-2192. [DOI] [PubMed] [Google Scholar]
- 37.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li KK, Powell NB, Kushida C, Riley RW, Adornato B, Guilleminault C. A comparison of Asian and white patients with obstructive sleep apnea syndrome. Laryngoscope. 1999;109:1937–1940. doi: 10.1097/00005537-199912000-00007. [DOI] [PubMed] [Google Scholar]