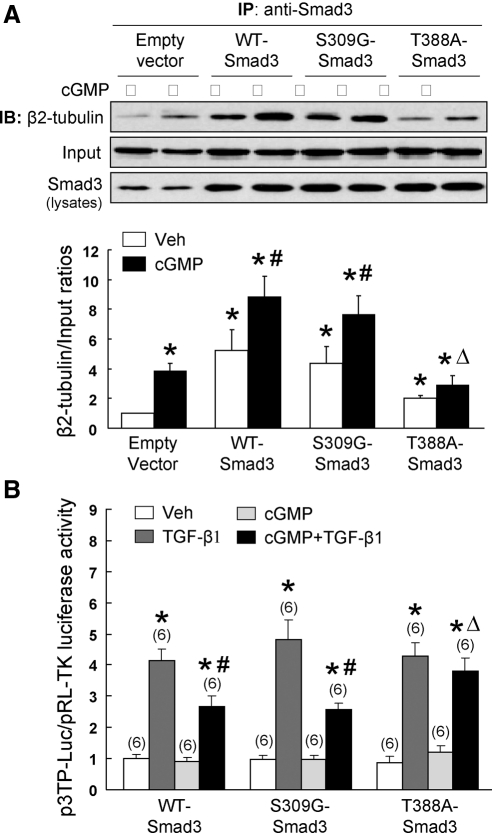
Fig. 4.
Mutation of Smad3 at the Thr388 residue attenuates cGMP-induced Smad3 binding to β2-tubulin and abolishes the inhibitory effect of cGMP on TGF-β-induced PAI-1 transcription. A, Serum-starved HEK293 cells were transiently transfected with wild-type Smad3 (WT-Smad3), S309G-Smad3, T388A-Smad3, or empty vector. After 72 h, serum-starved cells were treated with cGMP (0.5 mm) for 1 h. Whole cell extracts were precipitated with anti-Smad3 antibody and then were subjected to IB analysis using anti-β2-tubulin. After IB analysis, the blot was stripped and then probed with anti-Smad3 (input). Same amount of cellular lysate was loaded for IB analysis to detect Smad3 overexpression. Representative IB of the immunoprecititates from three independent experiments are shown. B, PASMC were cotransfected with wild-type and mutant Smad3 plasmids, p3TP-Luc, and pRL-TK. At 48 h after transfection, serum-starved cells were pretreated cGMP for 1 h and followed by TGF-β1 (2 ng/ml) for 24 h. Luciferase activity was measured. Results are means ± sem; n, Sample size. *, P < 0.05 vs. vehicle (Veh); #, P < 0.01 vs. TGF-β1; ▵, P < 0.01 vs. cGMP + TGF-β1 in WT-Smad3 group.