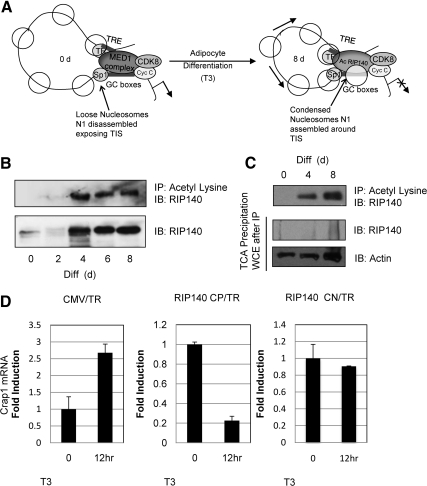
Fig. 1.
Acetylated RIP140 down-regulates Crabp1 mRNA levels in adipocyte differentiation. A, A model based upon our previous findings. In predifferentiated cultures, T3 initially activates crabp1 gene expression. The MED1 complex associated with factors CDK8 and cyclin C maintains the juxtaposed chromatin conformation between the distal TRE region through TRα, and through specificity protein-1 at the GC boxes. During this period of T3 activation, nucleosomes slide toward 3′-end resulting in disassembly of the nucleosome covering the TIS, allowing activation of the Crabp1 gene. After differentiation, MED1 and associated coactivators are no longer detected, instead, acetylated RIP140 and associated corepressors along with CDK8 and cyclin C are detected at the promoter and maintain the juxtaposed conformation. Arrows depict nucleosomes sliding bidirectionally toward the 5′-end and 3′-end of the loop to cover the entire promoter, the chromatin then adopts a more compact conformation, and the gene becomes silenced. B, Increase in lysine-acetylated RIP140 during adipocyte differentiation. Co-IP with acetyl lysine in differentiating 3T3-L1 cells to monitor acetylated RIP140 levels. RIP140 protein expression levels shown throughout differentiation in 3T3-L1. C, Ratio of acetylated/unacetylated RIP140 at differentiation d 0, 4, and 8 in 3T3-L1 cells. Co-IP with acetyl lysine (top panel) shows RIP140 acetylation status. TCA precipitation of the remaining whole cell extract (WCE) (after IP with acetyl lysine) reveals deacatylated pool, lacking RIP140 (middle panel). D, qPCR Crabp1 mRNA levels in undifferentiated 3T3-L1 transfected with human TRα and cytomegalovirus (CMV) promoter driven empty vector (left panel) RIP140 CP acetylation mutant (K158/287Q) (middle panel) or RIP140 CN acetylation mutant (K158/287A) with and without T3 treatment (12 h, 1 μm). IB, Immunoblot; CN, constitutive negative; CP, constitutive positive; Diff., differentiation.