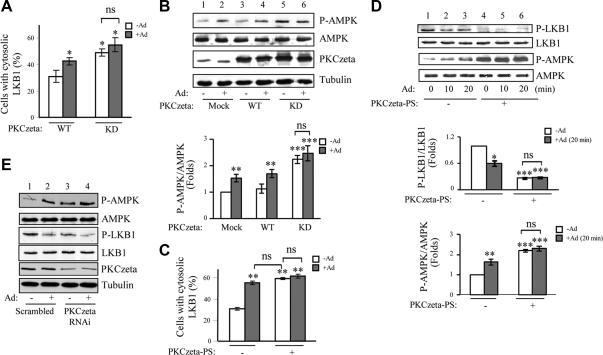
Fig. 3.
Inactivation of PKCζ induces cytosolic translocation of LKB1 and subsequent AMPK activation. A, The kinase activity of PKCζ is inversely related to adiponectin (Ad)-induced LKB1 cytosolic translocation. Localization of hemagglutinin-tagged LKB1 coexpressed with myc-tagged wild type (WT) or kinase inactive mutant (KD) of PKCζ in C2C12 myoblasts with or without adiponectin treatment (1 μg/ml, 20 min) was determined by confocal microscopy images with specific antibodies to the tags as described in Materials and Methods. The cells with cytosolic LKB1 were counted, analyzed, and shown as graphic representation. The error bars represent mean ± sem from three independent experiments. *, P < 0.05 vs. wild-type LKB1 without adiponectin treatment. ns, Nonsignificant difference in statistics. B, The kinase inactive mutant of PKCζ increases basal AMPK phosphorylation. C2C12 myoblasts were transiently transfected with myc-tagged wild-type or kinase inactive mutant (KD) of PKCζ and treated with or without adiponectin (1 μg/ml, 20 min). The phosphorylation of AMPK (top panel), the protein levels of AMPK (second panel) and PKCζ (third panel), and β-tubulin loading control (fourth panel) were detected by Western blot analysis with specific antibodies as indicated. The relative phosphorylation level of AMPK was shown as graphic representation. The error bars represent mean ± sem from three independent experiments. ** and ***, P < 0.01 and P < 0.001, respectively, vs. mock transfection without adiponectin treatment. C, PS of PKCζ blocked adiponectin-induced LKB1 translocation. Localization of myc-tagged LKB1 in C2C12 myoblasts with or without PKCζ-PS pretreatment (10 μm, 1 h) and with or without adiponectin (1 μg/ml, 20 min) stimulation was determined by confocal microscopy images with specific antibodies to the tags as described in Materials and Methods. The cells with cytosolic LKB1 were counted, analyzed, and shown as graphic representation. The error bars represent mean ± sem from three independent experiments. **, P < 0.01 vs. PKCζ-PS negative control without adiponectin treatment. D, Inhibition of PKCζ using PKCζ-PS increases basal AMPK phosphorylation. C2C12 myotubes were pretreated with or without PKCζ-PS (10 μm, 1 h) and treated with or without adiponectin (1 μg/ml, 20 min). LKB1 phosphorylation at Ser307 (first panel), AMPK phosphorylation at Thr172 (third panel), and protein levels of LKB1 (second panel) and AMPK (fourth panel) were determined by Western blot analysis with specific antibodies as indicated. The relative phosphorylation levels of LKB1 and AMPK were shown as graphic representations. The error bars represent mean ± sem from three independent experiments. *, **, and ***, P < 0.05, P < 0.01, and P < 0.001, respectively, vs. PKCζ-PS negative control without adiponectin treatment. E, Suppression of endogenous PKCζ expression enhances basal AMPK phosphorylation. shRNA PKCζ-suppressed or the control C2C12 myotubes were treated with or without adiponectin (1 μg/ml) for 20 min. Thr172 phosphorylation of AMPK (first panel), protein levels of AMPK (second panel), phosphorylation of LKB1 at Ser307 (third panel), protein levels of LKB1 (fourth panel) and PKCζ (fifth panel), and tubulin loading control (bottom panel) were detected by Western blot analysis with specific antibodies as indicated.