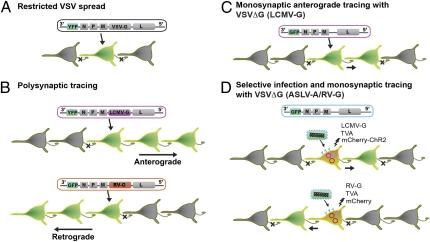
Fig. 1.
Schematic description of the transneuronal tracers generated by Beier et al. (3) in PNAS. (A) The VSV genome was modified such that a fluorescent reporter (YFP) was placed upstream of viral proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), G, and polymerase (L). VSV with endogenous G did not display transsynaptic spread. (B) Directionally selective polysynaptic tracers were generated by replacing the VSV-G with LCMV-G (purple) for anterograde spread, or with RV-G (orange) for retrograde spread of the viral vector. (C) G protein-deficient VSV (VSVΔG) pseudotyped with LCMV-G exhibited monosynaptic anterograde spread. (D) ASLV-A/RV-G pseudotyped VSVΔG can only infect the neurons expressing the TVA receptor from biolistic transfection (blue). By cotransfecting the gene for the LCMV-G in the same neurons, the VSVΔG can produce viruses that were able to transsynaptically infect postsynaptic neurons (Upper). VSVΔG repackaged with the G coat protein expressed from RV-G plasmid (orange) displayed transsynaptic spread to the presynaptic population of neurons (Lower). Because only the initially infected neuron contains virus G protein (LCMV-G or RV-G), VSV cannot spread any further, limiting VSV infection to neurons monosynaptically connected to initially infected population. A plasmid encoding mCherry was cotransfected for the cell originally targeted for infection to be identified within the monosynaptic network of GFP-labeled cells.